
Contributions
Abstract: EP526
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Tisagenlecleucel (Tisa-cel) is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Results from the JULIET trial (Schuster, NEJM 2019) showed best overall response of 52% per-protocol and 34% in an intention-to-treat (ITT) analysis. Outcomes of patients with out-of-commercial specification (OOS) products are not well established. The availability of point-of-care anti-CD19/CD28 costimulatory domain academic CAR T-cells in our center allows us to provide rapid salvage in cases of commercial CAR T-cell production failure.
Aims
To report real-world outcomes of patients treated at a single center with Tisa-cel for DLBCL and describe outcomes in cases of OOS in Tisa-cel production.
Methods
Data of patients who underwent leukapheresis for Tisa-cel were collected and analysed. Patients received lymphodepletion with fludarabine and cyclophosphamide. Survival was calculated from leukapheresis.
Results
Peripheral blood cells were collected from 37 patients with DLBCL between May 2019 and February 2020. The median age at leukapheresis was 63 (range 28-79) years. The median number of previous treatment lines was 2 (range 2-6). Cells from 78% of the patients were collected in progressive disease. Bridging chemotherapy was given in 87% of the patients. Median time from leukapheresis to cell infusion was 1.6 (range 1.3-2.2) months. During this time, disease progression was marked in 45% of the patients. Tisa-cel CAR T-cells were infused in 29 (78%) of the patients. The median follow-up was 14.7 (IQR: 13-17) months.
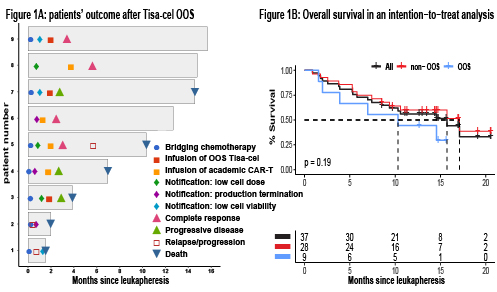
In 9/37 (24%) patients, manufactured Tisa-cel did not meet specification for commercial release. Causes for OOS were production termination (3/9 [33%]), low CAR T-cells viability (<70%; 3/9 [33%]), and low CAR T-cells dose (<0.6x108; 3/9 [33%]). Among patients with OOS products, 3/9 (33%) received Tisa-cel, despite not meeting production standards; 2/9 (22%) did not receive Tisa-cel due to disease progression and death before salvage therapy; and 4/9 (44%) were salvaged with point-of-care academic CD19-directed CAR T-cells, at a median of 35 (range 16-92) days following the notification of production failure. Three of these four patients achieved a complete response (CR) (Figure 1A). In a univariable analysis, risk factors for OOS were ≥ 4 previous lines of treatment (p=0.04) and previous exposure to bendamustine (p=0.04). Previous bone marrow transplantation, age ≥ 65, and time from previous last therapy were not significant risk factors.
Overall response at day-28 post-CAR-T was 46% (CR 24%) and 35% (CR 24%) in per-protocol (i.e., including only patients who received Tisa-cel) and ITT (i.e., including all patients who underwent leukapheresis) analysis, respectively. One year overall survival (OS) was 65% (95% CI 50-85%) in per-protocol and 56% (95% CI 42-75%) in ITT (Figure 1B). In a multivariable ITT Cox regression, factors associated with a shorter OS were ECOG ≥ 2 (HR 6.5; 95% CI 1.9-22.5) and primary refractory disease (HR 3.3; 95% CI 1.2-9.1). Age, disease stage at leukapheresis, and LDH at leukapheresis were not significant risk factors. OOS was not statistically significant as risk factor for a shorter OS (p=0.07).
Conclusion
Our data show relatively high rates of out-of-specification products following leukapheresis for Tisa-cel. We highlight the ability to rapidly produce point-of-care CAR T-cells, facilitating immune-effector cell salvage of patients who experienced production failure of the commercial product.
Keyword(s): CAR-T, Diffuse large B cell lymphoma, Lymphoma therapy, Outcome
Abstract: EP526
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Tisagenlecleucel (Tisa-cel) is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Results from the JULIET trial (Schuster, NEJM 2019) showed best overall response of 52% per-protocol and 34% in an intention-to-treat (ITT) analysis. Outcomes of patients with out-of-commercial specification (OOS) products are not well established. The availability of point-of-care anti-CD19/CD28 costimulatory domain academic CAR T-cells in our center allows us to provide rapid salvage in cases of commercial CAR T-cell production failure.
Aims
To report real-world outcomes of patients treated at a single center with Tisa-cel for DLBCL and describe outcomes in cases of OOS in Tisa-cel production.
Methods
Data of patients who underwent leukapheresis for Tisa-cel were collected and analysed. Patients received lymphodepletion with fludarabine and cyclophosphamide. Survival was calculated from leukapheresis.
Results
Peripheral blood cells were collected from 37 patients with DLBCL between May 2019 and February 2020. The median age at leukapheresis was 63 (range 28-79) years. The median number of previous treatment lines was 2 (range 2-6). Cells from 78% of the patients were collected in progressive disease. Bridging chemotherapy was given in 87% of the patients. Median time from leukapheresis to cell infusion was 1.6 (range 1.3-2.2) months. During this time, disease progression was marked in 45% of the patients. Tisa-cel CAR T-cells were infused in 29 (78%) of the patients. The median follow-up was 14.7 (IQR: 13-17) months.
In 9/37 (24%) patients, manufactured Tisa-cel did not meet specification for commercial release. Causes for OOS were production termination (3/9 [33%]), low CAR T-cells viability (<70%; 3/9 [33%]), and low CAR T-cells dose (<0.6x108; 3/9 [33%]). Among patients with OOS products, 3/9 (33%) received Tisa-cel, despite not meeting production standards; 2/9 (22%) did not receive Tisa-cel due to disease progression and death before salvage therapy; and 4/9 (44%) were salvaged with point-of-care academic CD19-directed CAR T-cells, at a median of 35 (range 16-92) days following the notification of production failure. Three of these four patients achieved a complete response (CR) (Figure 1A). In a univariable analysis, risk factors for OOS were ≥ 4 previous lines of treatment (p=0.04) and previous exposure to bendamustine (p=0.04). Previous bone marrow transplantation, age ≥ 65, and time from previous last therapy were not significant risk factors.
Overall response at day-28 post-CAR-T was 46% (CR 24%) and 35% (CR 24%) in per-protocol (i.e., including only patients who received Tisa-cel) and ITT (i.e., including all patients who underwent leukapheresis) analysis, respectively. One year overall survival (OS) was 65% (95% CI 50-85%) in per-protocol and 56% (95% CI 42-75%) in ITT (Figure 1B). In a multivariable ITT Cox regression, factors associated with a shorter OS were ECOG ≥ 2 (HR 6.5; 95% CI 1.9-22.5) and primary refractory disease (HR 3.3; 95% CI 1.2-9.1). Age, disease stage at leukapheresis, and LDH at leukapheresis were not significant risk factors. OOS was not statistically significant as risk factor for a shorter OS (p=0.07).

Conclusion
Our data show relatively high rates of out-of-specification products following leukapheresis for Tisa-cel. We highlight the ability to rapidly produce point-of-care CAR T-cells, facilitating immune-effector cell salvage of patients who experienced production failure of the commercial product.
Keyword(s): CAR-T, Diffuse large B cell lymphoma, Lymphoma therapy, Outcome


