
Contributions
Abstract: EP512
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Central nervous system (CNS) relapse occurs in 2-10% of DLBCL patients but carries a poor prognosis. Prophylactic intravenous high-dose methotrexate (HD-MTX) is recommended for patients with risk factors of CNS relapse, which requires hospital admission and increases the risk of treatment-related toxicity. However, there is limited evidence to guide whether it should be intercalated between cycles or given at the end of induction chemotherapy (EOI), and whether the optimal dosage of MTX is different between different risk groups.
Aims
To compare the toxicity and preventive efficacy on CNS relapse of intercalated HD-MTX with EOI delivery, and explore the optimal dosage of MTX for patients of different risk groups.
Methods
We conducted a retrospective analysis of 275 DLBCL patients from Sun Yat-sen University Cancer Center who received prophylactic HD-MTX with either intercalated (n=218) or EOI HD-MTX (n=57). We stratified the patients into two risk groups: patients with CNS-IPI 4-6, and/or testis involvement, and/or double hit lymphoma in the high risk group, and others in the low-intermediate risk group. Multivariable analyses (MVA) of risk factors for toxicity and delays were performed. The log-rank test and χ2 test was applied to determine factors associated with CNS relapse, progression-free survival (PFS), and overall survival (OS).
Results
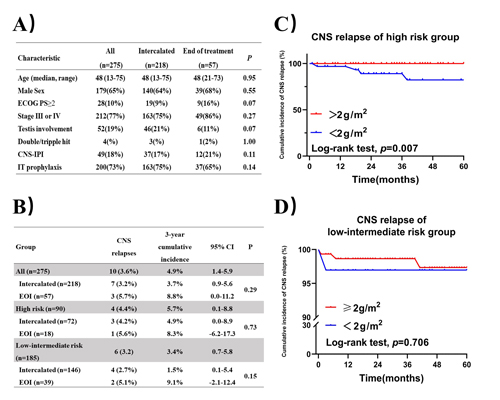
Figure 1A describes selected baseline characteristics. A total of 939 doses of intercalated HD-MTX and 127 doses of EOI HD-MTX was given. The treatment related adverse events were mainly hematological toxicity, including leucopenia (27%), neutropenia (20%) and thrombocytopenia (6%). Non-hematological toxicity mainly included gastrointestinal reactions (6%) and mucositis (3%). Nephrotoxicity was uncommon in this set (n=2, 1%). 197 (21%) intercalated HD-MTX cycles were associated with grade 3/4 toxicity, compared with 3 (2%) of EOI HD-MTX cycles (p<0.001). Intercalated HD-MTX was the only factor associated with grade 3/4 toxicity (HR 5.28(95% CI 2.11-13.23), p<0.001) and subsequent chemotherapy delays (HR 4.27(95% CI 2.45-7.45), p<0.001) on MVA. MVA also identified that intercalated HD-MTX delivery (HR 0.41 (95%CI 0.17-0.98), p=0.04) and induction therapy without rituximab (HR 0.37 (95%CI 0.16-0.88), p=0.03) were the significant factors negatively associated with complete remission (CR) rate of induction therapy. Overall, ten CNS relapses occurred (median time to relapse 17 months). The 3 year cumulative incidence of CNS relapse showed no significant difference between two kinds of MTX delivery either in all patients or in two risk groups (Figure 1B). There was no difference in PFS or OS either in all patients or in two risk groups (median follow-up 36.0 months). In addition, the optimal dosage of MTX was discussed. Relatively high MTX dose (≥2g/m2) decreased CNS relapse(Figure 1C) and improved the PFS (p=0.001) and OS (p=0.009) in high risk patients significantly. However, there was no significant difference in CNS relapse(Figure 1D) and survival outcomes between different dosage groups in low-intermediate risk patients.
Conclusion
Intercalated HD-MTX was associated with increased toxicity and subsequent chemotherapy delays compared to EOI delivery, which may decrease the efficacy of induction therapy. However, there was no significant difference of CNS relapse and survival outcomes between two kinds of MTX delivery, even in high risk group. For low-intermediate risk patients, high dosage of MTX may not improve efficacy of CNS prophylaxis or bring additional survival benefit compared with low dosage.
Keyword(s): CNS, CNS lymphoma, DLBCL, Methotrexate
Abstract: EP512
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Central nervous system (CNS) relapse occurs in 2-10% of DLBCL patients but carries a poor prognosis. Prophylactic intravenous high-dose methotrexate (HD-MTX) is recommended for patients with risk factors of CNS relapse, which requires hospital admission and increases the risk of treatment-related toxicity. However, there is limited evidence to guide whether it should be intercalated between cycles or given at the end of induction chemotherapy (EOI), and whether the optimal dosage of MTX is different between different risk groups.
Aims
To compare the toxicity and preventive efficacy on CNS relapse of intercalated HD-MTX with EOI delivery, and explore the optimal dosage of MTX for patients of different risk groups.
Methods
We conducted a retrospective analysis of 275 DLBCL patients from Sun Yat-sen University Cancer Center who received prophylactic HD-MTX with either intercalated (n=218) or EOI HD-MTX (n=57). We stratified the patients into two risk groups: patients with CNS-IPI 4-6, and/or testis involvement, and/or double hit lymphoma in the high risk group, and others in the low-intermediate risk group. Multivariable analyses (MVA) of risk factors for toxicity and delays were performed. The log-rank test and χ2 test was applied to determine factors associated with CNS relapse, progression-free survival (PFS), and overall survival (OS).
Results
Figure 1A describes selected baseline characteristics. A total of 939 doses of intercalated HD-MTX and 127 doses of EOI HD-MTX was given. The treatment related adverse events were mainly hematological toxicity, including leucopenia (27%), neutropenia (20%) and thrombocytopenia (6%). Non-hematological toxicity mainly included gastrointestinal reactions (6%) and mucositis (3%). Nephrotoxicity was uncommon in this set (n=2, 1%). 197 (21%) intercalated HD-MTX cycles were associated with grade 3/4 toxicity, compared with 3 (2%) of EOI HD-MTX cycles (p<0.001). Intercalated HD-MTX was the only factor associated with grade 3/4 toxicity (HR 5.28(95% CI 2.11-13.23), p<0.001) and subsequent chemotherapy delays (HR 4.27(95% CI 2.45-7.45), p<0.001) on MVA. MVA also identified that intercalated HD-MTX delivery (HR 0.41 (95%CI 0.17-0.98), p=0.04) and induction therapy without rituximab (HR 0.37 (95%CI 0.16-0.88), p=0.03) were the significant factors negatively associated with complete remission (CR) rate of induction therapy. Overall, ten CNS relapses occurred (median time to relapse 17 months). The 3 year cumulative incidence of CNS relapse showed no significant difference between two kinds of MTX delivery either in all patients or in two risk groups (Figure 1B). There was no difference in PFS or OS either in all patients or in two risk groups (median follow-up 36.0 months). In addition, the optimal dosage of MTX was discussed. Relatively high MTX dose (≥2g/m2) decreased CNS relapse(Figure 1C) and improved the PFS (p=0.001) and OS (p=0.009) in high risk patients significantly. However, there was no significant difference in CNS relapse(Figure 1D) and survival outcomes between different dosage groups in low-intermediate risk patients.

Conclusion
Intercalated HD-MTX was associated with increased toxicity and subsequent chemotherapy delays compared to EOI delivery, which may decrease the efficacy of induction therapy. However, there was no significant difference of CNS relapse and survival outcomes between two kinds of MTX delivery, even in high risk group. For low-intermediate risk patients, high dosage of MTX may not improve efficacy of CNS prophylaxis or bring additional survival benefit compared with low dosage.
Keyword(s): CNS, CNS lymphoma, DLBCL, Methotrexate


