
Contributions
Abstract: EP511
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Checkpoint blockade may complicate response assessment by the development of a flare reaction or pseudoprogression (PP). To avoid the mistake of considering a new agent ineffective, it was proposed to add the category called “indeterminate response” (IR) to Lugano classification, in the LYmphoma Response to Immunomodulatory Therapy Criteria (LYRIC): 1/ increase in overall tumor burden in the first 12 weeks of therapy, without clinical deterioration (IR1), 2/ development of new lesions without overall progression (IR2), or 3/ increased F-fluorodeoxyglucose avidity despite no change in lesion size (IR3). These 3 patterns are defined as progressive metabolic disease (PMD) in the Lugano criteria. A PP was defined as an IR that became a partial or complete metabolic response (PMR or CMR) in comparison to baseline, at next evaluation.
Aims
The aim of this study is to assess in a practical and prospective manner the LYRIC criteria in relapsed/refractory diffuse large B cell lymphomas (DLBCL) and follicular lymphomas (FL) treated with atezolizumab, venetoclax and obinutuzumab (GATA trial), as well as measuring the incidence of PP.
Methods
The GATA study is a LYSA sponsored multicenter phase 2 trial (NCT03276468) evaluating the combination of atezolizumab, obinutuzumab and venetoclax in biopsy-confirmed R/R DLBCL and FL patients who failed at least one line of therapy (rituximab and anthracycline containing regimen). Local imaging practitioners were invited to use the LYRIC classification. Clinical investigators were warned of the possibility of PP, and a coordinating investigator's approval was required before stopping treatment. A centralized imaging review on Positron Emission Tomography (PET) and Computed Tomography (CT) scan was performed at three time points: baseline, cycle 4 (C4, 12 weeks) and cycle 8 (C8, 24 weeks), on Lugano and LYRIC criteria.
Results
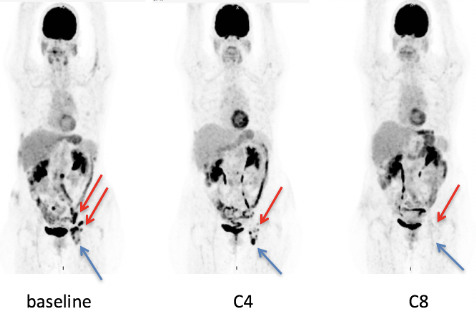
Our study has included 116 patients (58 DLBCL and 58 FL) from GATA trial. With a median follow-up of 9 months the overall metabolic response rate (OMRR) at C8 was measured at 23.6% for DLBCL patients. With a median follow-up of 14.5 months the OMRR at C8 was measured at 53.6% for FL patients. Ninety-five patients had a centralized review at C4 and 57 at C8. Missing patients are those who progressed prior to the restaging by PET and CT scan. Lugano criteria: At C4, 84 reviewed responses (88.4%) were concordant with local response. At C8, 47 reviewed responses (82.5%) were concordant with local response. The differences were mainly between NMR (no MR) and PMR. LYRIC criteria: At C4, 9 PMD were classified as IR by both local reading and centralized review, 4 DLBCL and 5 FL. A discordance in the type of IR (1, 2 or 3) was seen in 6 out of 9 cases. During the follow-up, 4 cases turned out to be PP (1 DLBCL and 3 FL) and 5 cases real PD. One of the PP is illustrated on Figure 1, with IR3 at C4 (both locally and centrally), and CMR at C8. At C8, 7 PMD were classified as IR by centralized review (IR2 and IR3), only 2 of them were IR by local reading. During the follow-up, all of those cases turned-out to be real progressive diseases within 1 to 3 cycles of therapy.
Conclusion
We did observe 3.4% of PP, all classified as IR at C4. A high frequency of discrepancy between local and centralized reading was observed with LYRIC criteria, probably due to the novelty of this classification. Interestingly, all IRs at C8 were found to be real progressions. Overall, these data are in favor of using the LYRIC classification, but only for early evaluation.
Keyword(s): Immunotherapy, Lymphoma, PET
Abstract: EP511
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Checkpoint blockade may complicate response assessment by the development of a flare reaction or pseudoprogression (PP). To avoid the mistake of considering a new agent ineffective, it was proposed to add the category called “indeterminate response” (IR) to Lugano classification, in the LYmphoma Response to Immunomodulatory Therapy Criteria (LYRIC): 1/ increase in overall tumor burden in the first 12 weeks of therapy, without clinical deterioration (IR1), 2/ development of new lesions without overall progression (IR2), or 3/ increased F-fluorodeoxyglucose avidity despite no change in lesion size (IR3). These 3 patterns are defined as progressive metabolic disease (PMD) in the Lugano criteria. A PP was defined as an IR that became a partial or complete metabolic response (PMR or CMR) in comparison to baseline, at next evaluation.
Aims
The aim of this study is to assess in a practical and prospective manner the LYRIC criteria in relapsed/refractory diffuse large B cell lymphomas (DLBCL) and follicular lymphomas (FL) treated with atezolizumab, venetoclax and obinutuzumab (GATA trial), as well as measuring the incidence of PP.
Methods
The GATA study is a LYSA sponsored multicenter phase 2 trial (NCT03276468) evaluating the combination of atezolizumab, obinutuzumab and venetoclax in biopsy-confirmed R/R DLBCL and FL patients who failed at least one line of therapy (rituximab and anthracycline containing regimen). Local imaging practitioners were invited to use the LYRIC classification. Clinical investigators were warned of the possibility of PP, and a coordinating investigator's approval was required before stopping treatment. A centralized imaging review on Positron Emission Tomography (PET) and Computed Tomography (CT) scan was performed at three time points: baseline, cycle 4 (C4, 12 weeks) and cycle 8 (C8, 24 weeks), on Lugano and LYRIC criteria.
Results
Our study has included 116 patients (58 DLBCL and 58 FL) from GATA trial. With a median follow-up of 9 months the overall metabolic response rate (OMRR) at C8 was measured at 23.6% for DLBCL patients. With a median follow-up of 14.5 months the OMRR at C8 was measured at 53.6% for FL patients. Ninety-five patients had a centralized review at C4 and 57 at C8. Missing patients are those who progressed prior to the restaging by PET and CT scan. Lugano criteria: At C4, 84 reviewed responses (88.4%) were concordant with local response. At C8, 47 reviewed responses (82.5%) were concordant with local response. The differences were mainly between NMR (no MR) and PMR. LYRIC criteria: At C4, 9 PMD were classified as IR by both local reading and centralized review, 4 DLBCL and 5 FL. A discordance in the type of IR (1, 2 or 3) was seen in 6 out of 9 cases. During the follow-up, 4 cases turned out to be PP (1 DLBCL and 3 FL) and 5 cases real PD. One of the PP is illustrated on Figure 1, with IR3 at C4 (both locally and centrally), and CMR at C8. At C8, 7 PMD were classified as IR by centralized review (IR2 and IR3), only 2 of them were IR by local reading. During the follow-up, all of those cases turned-out to be real progressive diseases within 1 to 3 cycles of therapy.

Conclusion
We did observe 3.4% of PP, all classified as IR at C4. A high frequency of discrepancy between local and centralized reading was observed with LYRIC criteria, probably due to the novelty of this classification. Interestingly, all IRs at C8 were found to be real progressions. Overall, these data are in favor of using the LYRIC classification, but only for early evaluation.
Keyword(s): Immunotherapy, Lymphoma, PET


