
Contributions
Abstract: EP510
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
The efficacy of chimeric antigen receptor T-cell therapies tisa-cel and liso-cel as effective treatments for r/r DLBCL was demonstrated in JULIET and TRANSCEND (Schuster 2019, Abramson 2020), respectively.
Aims
To compare efficacy outcomes of tisa-cel and liso-cel in r/r DLBCL using matching-adjusted indirect comparison (MAIC).
Methods
Individual patient-level data (IPD) from JULIET (tisa-cel; NCT02445248; 02/2020 datacut) were weighted to match the patient population in TRANSCEND (liso-cel; NCT02631044; 08/2019 datacut). Baseline prognostic factors available in both trials were adjusted for age, sex, histology, ECOG performance status (ECOG PS), left ventricular ejection fraction, radiologic sum of product diameters, lactate dehydrogenase, prior stem cell transplantation (SCT), use of bridging therapy, and number of and refractoriness to prior therapies in the MAIC. Overall survival (OS), progression-free survival (PFS), complete response (CR) rate, and overall response (OR) rate were compared. Primary analyses compared infused patients in JULIET (N=106, excluding 8 without lymphodepleting chemotherapy [LDC] and 1 large cell neuroendocrine carcinoma) with the efficacy-evaluable set in TRANSCEND (N=256, infused patients). A scenario analysis compared JULIET infused to the TRANSCEND primary analysis set (PAS) (N=133, dose level 2, excluding those with ECOG PS 2, prior allogeneic SCT, primary mediastinal B-cell lymphoma, follicular lymphoma [FL] 3B, or transformation from indolent lymphoma besides FL). Sensitivity analyses included JULIET patients with only fludarabine-based LDC or only adjusted significantly different baseline prognostic factors. Safety outcomes were not compared because adverse event management has evolved and differed between the two trials; MAIC is unable to adjust for such differences.
Results
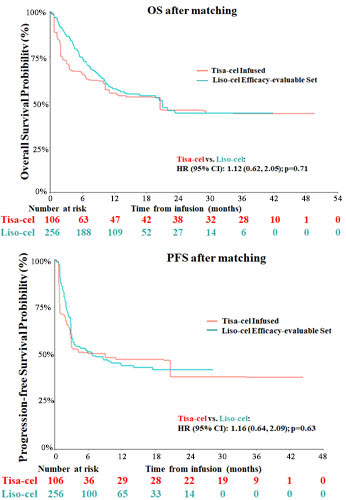
After adjusting for differences in baseline characteristics, no significant differences were observed in OS (hazard ratio [95% confidence interval (CI)]: 1.12 [0.62, 2.05], p=0.71) or PFS (1.16 [0.64, 2.09], p=0.63) between tisa-cel infused patients and the liso-cel efficacy-evaluable set (Figure). CR was also comparable (rate difference [95% CI]: -5.4% [-15.5%, 4.7%], p=0.29). The results comparing OS, PFS, and CR were consistent across all scenario and sensitivity analyses. The OR rate trended higher in the TRANSCEND efficacy-evaluable set (-9.7% [-20.0%, 0.6%], p=0.07) and was higher in the TRANSCEND PAS than in the respectively matched JULIET infused set (-13.5% [-25.6%, -1.5%], p<0.05).
Conclusion
The MAIC results indicate there is no evidence suggesting differences in OS, PFS, and CR between tisa-cel and liso-cel in r/r DLBCL. Analyses using IPD from both trials and/or real-world evidence are warranted to confirm these findings.
Keyword(s): CAR-T, Diffuse large B cell lymphoma, Relapsed lymphoma
Abstract: EP510
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
The efficacy of chimeric antigen receptor T-cell therapies tisa-cel and liso-cel as effective treatments for r/r DLBCL was demonstrated in JULIET and TRANSCEND (Schuster 2019, Abramson 2020), respectively.
Aims
To compare efficacy outcomes of tisa-cel and liso-cel in r/r DLBCL using matching-adjusted indirect comparison (MAIC).
Methods
Individual patient-level data (IPD) from JULIET (tisa-cel; NCT02445248; 02/2020 datacut) were weighted to match the patient population in TRANSCEND (liso-cel; NCT02631044; 08/2019 datacut). Baseline prognostic factors available in both trials were adjusted for age, sex, histology, ECOG performance status (ECOG PS), left ventricular ejection fraction, radiologic sum of product diameters, lactate dehydrogenase, prior stem cell transplantation (SCT), use of bridging therapy, and number of and refractoriness to prior therapies in the MAIC. Overall survival (OS), progression-free survival (PFS), complete response (CR) rate, and overall response (OR) rate were compared. Primary analyses compared infused patients in JULIET (N=106, excluding 8 without lymphodepleting chemotherapy [LDC] and 1 large cell neuroendocrine carcinoma) with the efficacy-evaluable set in TRANSCEND (N=256, infused patients). A scenario analysis compared JULIET infused to the TRANSCEND primary analysis set (PAS) (N=133, dose level 2, excluding those with ECOG PS 2, prior allogeneic SCT, primary mediastinal B-cell lymphoma, follicular lymphoma [FL] 3B, or transformation from indolent lymphoma besides FL). Sensitivity analyses included JULIET patients with only fludarabine-based LDC or only adjusted significantly different baseline prognostic factors. Safety outcomes were not compared because adverse event management has evolved and differed between the two trials; MAIC is unable to adjust for such differences.
Results
After adjusting for differences in baseline characteristics, no significant differences were observed in OS (hazard ratio [95% confidence interval (CI)]: 1.12 [0.62, 2.05], p=0.71) or PFS (1.16 [0.64, 2.09], p=0.63) between tisa-cel infused patients and the liso-cel efficacy-evaluable set (Figure). CR was also comparable (rate difference [95% CI]: -5.4% [-15.5%, 4.7%], p=0.29). The results comparing OS, PFS, and CR were consistent across all scenario and sensitivity analyses. The OR rate trended higher in the TRANSCEND efficacy-evaluable set (-9.7% [-20.0%, 0.6%], p=0.07) and was higher in the TRANSCEND PAS than in the respectively matched JULIET infused set (-13.5% [-25.6%, -1.5%], p<0.05).

Conclusion
The MAIC results indicate there is no evidence suggesting differences in OS, PFS, and CR between tisa-cel and liso-cel in r/r DLBCL. Analyses using IPD from both trials and/or real-world evidence are warranted to confirm these findings.
Keyword(s): CAR-T, Diffuse large B cell lymphoma, Relapsed lymphoma


