
Contributions
Abstract: EP509
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Cutaneous T-cell lymphomas (CTCL) are rare, serious and potentially life-threatening forms of non-Hodgkin lymphoma that primarily present in skin. Mycosis fungoides (MF) and Sézary syndrome (SS) are the classic subtypes and together account for around two-thirds of all CTCLs. Initial methods of disease staging in MF and SS built upon the tumour-node-metastasis (TNM) classification using disease-specific findings. Blood classification (B0–2) was added to staging in 2007 based upon the recognition of blood involvement as a prognostic factor; increasing blood tumour burden has previously been linked with worsening of overall survival (OS) and disease-specific survival (DSS), and an increased risk of disease progression (RDP) (Agar 2010, Am Soc J Clin Oncol), although this is a subject of debate and further study. Patients with B1 disease have previously been shown to have a median survival of just 3.2 years, similar to B2 for which it was 3.1 years (Agar 2010, Am Soc J Clin Oncol). The addition of blood classification allows for more specific disease staging which may inform clinical management strategy, whilst also contributing to a better understanding of prognostic factors and treatment response in MF and SS.
Aims
This post hoc analysis examined the efficacy and safety of mogamulizumab (MOGA) compared with vorinostat (VORI), stratified by patient blood classification.
Methods
MAVORIC (NCT01728805) was an open-label, phase 3 study where patients were randomized 1:1 to receive either intravenous MOGA 1.0 mg/kg weekly for the first 28 day cycle, then on days 1 and 15 of subsequent cycles, or oral VORI 400 mg once daily. VORI patients who experienced disease progression or intolerable toxicity could cross over to MOGA. The primary endpoint was investigator-assessed progression-free survival (PFS).
Results
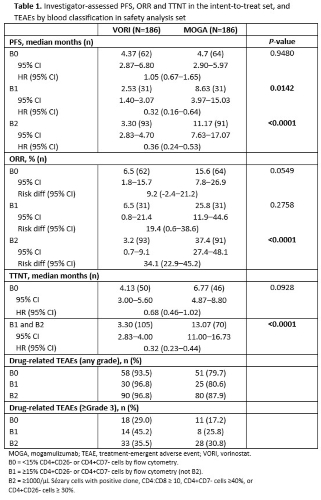
In MAVORIC, investigator-assessed PFS was significantly greater for MOGA than VORI overall at 7.7 months and 3.1 months, respectively (P<0.0001). When data were stratified by blood classification, PFS was found to be significantly superior for MOGA as compared to VORI in patients with both B1 and B2 disease (Table 1). Overall response rate (ORR) was also significantly greater for MOGA than VORI in MAVORIC at 28.0% and 4.8% respectively (P<0.0001), and was found in this analysis to be significantly greater for MOGA than VORI in those patients with B2 disease (Table 1). ORR for B1 was not significant, but showed a trend (25.8% vs 6.5% for MOGA and VORI, respectively). Time-to-next-treatment (TTNT) was not significant for patients without blood involvement (B0), but was significantly greater for MOGA in patients with blood involvement (B1 or B2) with 13.70 and 3.30 months for MOGA and VORI respectively (P<0.0001) (Table 1). Drug-related treatment-emergent adverse events (TEAEs) were similar in patients regardless of blood involvement and were lower for MOGA than VORI at each blood classification level (Table 1).
Conclusion
MOGA is effective in patients with blood involvement (B1 and B2), including patients with MF. Drug safety is similar between patients irrespective of level of blood involvement.
Keyword(s): Clinical data, Cutaneous T-cell lymphoma, Mycosis fungoides, Peripheral blood
Abstract: EP509
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Cutaneous T-cell lymphomas (CTCL) are rare, serious and potentially life-threatening forms of non-Hodgkin lymphoma that primarily present in skin. Mycosis fungoides (MF) and Sézary syndrome (SS) are the classic subtypes and together account for around two-thirds of all CTCLs. Initial methods of disease staging in MF and SS built upon the tumour-node-metastasis (TNM) classification using disease-specific findings. Blood classification (B0–2) was added to staging in 2007 based upon the recognition of blood involvement as a prognostic factor; increasing blood tumour burden has previously been linked with worsening of overall survival (OS) and disease-specific survival (DSS), and an increased risk of disease progression (RDP) (Agar 2010, Am Soc J Clin Oncol), although this is a subject of debate and further study. Patients with B1 disease have previously been shown to have a median survival of just 3.2 years, similar to B2 for which it was 3.1 years (Agar 2010, Am Soc J Clin Oncol). The addition of blood classification allows for more specific disease staging which may inform clinical management strategy, whilst also contributing to a better understanding of prognostic factors and treatment response in MF and SS.
Aims
This post hoc analysis examined the efficacy and safety of mogamulizumab (MOGA) compared with vorinostat (VORI), stratified by patient blood classification.
Methods
MAVORIC (NCT01728805) was an open-label, phase 3 study where patients were randomized 1:1 to receive either intravenous MOGA 1.0 mg/kg weekly for the first 28 day cycle, then on days 1 and 15 of subsequent cycles, or oral VORI 400 mg once daily. VORI patients who experienced disease progression or intolerable toxicity could cross over to MOGA. The primary endpoint was investigator-assessed progression-free survival (PFS).
Results
In MAVORIC, investigator-assessed PFS was significantly greater for MOGA than VORI overall at 7.7 months and 3.1 months, respectively (P<0.0001). When data were stratified by blood classification, PFS was found to be significantly superior for MOGA as compared to VORI in patients with both B1 and B2 disease (Table 1). Overall response rate (ORR) was also significantly greater for MOGA than VORI in MAVORIC at 28.0% and 4.8% respectively (P<0.0001), and was found in this analysis to be significantly greater for MOGA than VORI in those patients with B2 disease (Table 1). ORR for B1 was not significant, but showed a trend (25.8% vs 6.5% for MOGA and VORI, respectively). Time-to-next-treatment (TTNT) was not significant for patients without blood involvement (B0), but was significantly greater for MOGA in patients with blood involvement (B1 or B2) with 13.70 and 3.30 months for MOGA and VORI respectively (P<0.0001) (Table 1). Drug-related treatment-emergent adverse events (TEAEs) were similar in patients regardless of blood involvement and were lower for MOGA than VORI at each blood classification level (Table 1).

Conclusion
MOGA is effective in patients with blood involvement (B1 and B2), including patients with MF. Drug safety is similar between patients irrespective of level of blood involvement.
Keyword(s): Clinical data, Cutaneous T-cell lymphoma, Mycosis fungoides, Peripheral blood


