
Contributions
Abstract: EP506
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
ECHELON-2 (NCT01777152), a phase 3, randomised, double-blind, double-dummy, placebo-controlled, active-comparator, multicentre study, established the superiority of frontline brentuximab vedotin + cyclophosphamide, doxorubicin, and prednisone (A+CHP) vs cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) for the treatment of patients (pts) with systemic anaplastic large cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCLs) (Horwitz, Lancet 2019). Both risk of progression-free survival (PFS) per blinded independent central review (primary endpoint) and overall survival (OS) events favoured A+CHP over CHOP at the primary analysis. A+CHP was the first treatment regimen to increase OS compared with CHOP in this population.
Aims
We report the 5-year data from ECHELON-2, including PFS per investigator (INV) data and the following key secondary endpoints: OS, PFS in sALCL, complete remission (CR) rate, and objective response rate (ORR) in re-treated pts.
Methods
Adults with untreated CD30-positive PTCL (targeting 75% ± 5% with sALCL) were randomized 1:1 to receive 6–8 cycles of A+CHP or CHOP. Pts were stratified by histological subtype and international prognostic index (IPI) score. Brentuximab vedotin-based subsequent therapies were allowed.
Results
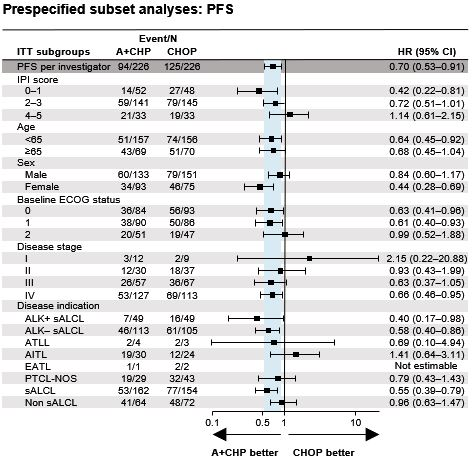
Of 452 pts enrolled, the majority had sALCL (n=316 [70%]; 218 [48%] anaplastic lymphoma kinase [ALK]-negative, and 98 pts [22%] ALK-positive) and had advanced disease (27% Stage III, 53% Stage IV; 78% IPI ≥2). At data cutoff, median follow-up was 47.6 months for PFS and 66.8 months for OS. A+CHP was favoured over CHOP with a hazard ratio (HR) for PFS per INV of 0.70 (95% confidence interval [CI]: 0.53, 0.91; p=0.0077) and OS HR of 0.72 (95% CI: 0.53, 0.99; p=0.0424). Median PFS was 62.3 months (95% CI: 42.0, not evaluable) for A+CHP, and 23.8 months (95% CI: 13.6, 60.8) for CHOP. Estimated 5-year PFS was 51.4% (95% CI: 42.8, 59.4) and 43.0% (95% CI: 35.8, 50.0) with A+CHP and CHOP, respectively. Median OS was not reached in either arm. Estimated 5-year OS was 70.1% (95% CI: 63.3, 75.9) for A+CHP vs 61.0% (95% CI: 54.0, 67.3) for CHOP. PFS in prespecified subgroups and overall PFS were generally consistent (Figure). The HR for PFS (0.55 [95% CI: 0.39, 0.79]) also favoured A+CHP over CHOP in pts with sALCL, with an estimated 5-year PFS of 60.6% (95% CI: 49.5, 69.9) for the A+CHP arm vs 48.4% (95% CI: 39.6, 56.7) for the CHOP arm. Subsequent systemic therapy with brentuximab vedotin was administered to a total of 29 pts (13%) in the A+CHP arm (sALCL [n=19]; PTCL not otherwise specified [n=5], angioimmunoblastic T-cell lymphoma [n=5]) and 54 pts (24%) in the CHOP arm. Median time to retreatment for pts in the A+CHP arm was 15.0 months (range, 3–64); 17 pts (ORR: 59%) had CR (n=11) or partial remission (n=6) after retreatment with brentuximab vedotin monotherapy (n=25) or a brentuximab vedotin-containing regimen (n=4). Of the treatment-emergent peripheral neuropathy (PN) in the A+CHP (n=117) and CHOP arms (n=124), 72% in the A+CHP arm and 78% in the CHOP arm had resolved or improved. In pts with ongoing events at last follow-up (A+CHP [n=47] vs CHOP [n=42]) PN was grade 1, 2 and 3 in 70% vs 71%, 28% vs 26% and 2% vs 2%, respectively.
Conclusion
After 5 years’ follow-up, frontline A+CHP continued to provide clinically meaningful improvements in PFS and OS vs CHOP, including sustained remission in 59% of re-treated pts with sALCL, as well as a manageable safety profile, including continued resolution or improvement of PN.
Keyword(s): CD30, Phase III, T cell lymphoma, Targeted therapy
Abstract: EP506
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
ECHELON-2 (NCT01777152), a phase 3, randomised, double-blind, double-dummy, placebo-controlled, active-comparator, multicentre study, established the superiority of frontline brentuximab vedotin + cyclophosphamide, doxorubicin, and prednisone (A+CHP) vs cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) for the treatment of patients (pts) with systemic anaplastic large cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCLs) (Horwitz, Lancet 2019). Both risk of progression-free survival (PFS) per blinded independent central review (primary endpoint) and overall survival (OS) events favoured A+CHP over CHOP at the primary analysis. A+CHP was the first treatment regimen to increase OS compared with CHOP in this population.
Aims
We report the 5-year data from ECHELON-2, including PFS per investigator (INV) data and the following key secondary endpoints: OS, PFS in sALCL, complete remission (CR) rate, and objective response rate (ORR) in re-treated pts.
Methods
Adults with untreated CD30-positive PTCL (targeting 75% ± 5% with sALCL) were randomized 1:1 to receive 6–8 cycles of A+CHP or CHOP. Pts were stratified by histological subtype and international prognostic index (IPI) score. Brentuximab vedotin-based subsequent therapies were allowed.
Results
Of 452 pts enrolled, the majority had sALCL (n=316 [70%]; 218 [48%] anaplastic lymphoma kinase [ALK]-negative, and 98 pts [22%] ALK-positive) and had advanced disease (27% Stage III, 53% Stage IV; 78% IPI ≥2). At data cutoff, median follow-up was 47.6 months for PFS and 66.8 months for OS. A+CHP was favoured over CHOP with a hazard ratio (HR) for PFS per INV of 0.70 (95% confidence interval [CI]: 0.53, 0.91; p=0.0077) and OS HR of 0.72 (95% CI: 0.53, 0.99; p=0.0424). Median PFS was 62.3 months (95% CI: 42.0, not evaluable) for A+CHP, and 23.8 months (95% CI: 13.6, 60.8) for CHOP. Estimated 5-year PFS was 51.4% (95% CI: 42.8, 59.4) and 43.0% (95% CI: 35.8, 50.0) with A+CHP and CHOP, respectively. Median OS was not reached in either arm. Estimated 5-year OS was 70.1% (95% CI: 63.3, 75.9) for A+CHP vs 61.0% (95% CI: 54.0, 67.3) for CHOP. PFS in prespecified subgroups and overall PFS were generally consistent (Figure). The HR for PFS (0.55 [95% CI: 0.39, 0.79]) also favoured A+CHP over CHOP in pts with sALCL, with an estimated 5-year PFS of 60.6% (95% CI: 49.5, 69.9) for the A+CHP arm vs 48.4% (95% CI: 39.6, 56.7) for the CHOP arm. Subsequent systemic therapy with brentuximab vedotin was administered to a total of 29 pts (13%) in the A+CHP arm (sALCL [n=19]; PTCL not otherwise specified [n=5], angioimmunoblastic T-cell lymphoma [n=5]) and 54 pts (24%) in the CHOP arm. Median time to retreatment for pts in the A+CHP arm was 15.0 months (range, 3–64); 17 pts (ORR: 59%) had CR (n=11) or partial remission (n=6) after retreatment with brentuximab vedotin monotherapy (n=25) or a brentuximab vedotin-containing regimen (n=4). Of the treatment-emergent peripheral neuropathy (PN) in the A+CHP (n=117) and CHOP arms (n=124), 72% in the A+CHP arm and 78% in the CHOP arm had resolved or improved. In pts with ongoing events at last follow-up (A+CHP [n=47] vs CHOP [n=42]) PN was grade 1, 2 and 3 in 70% vs 71%, 28% vs 26% and 2% vs 2%, respectively.

Conclusion
After 5 years’ follow-up, frontline A+CHP continued to provide clinically meaningful improvements in PFS and OS vs CHOP, including sustained remission in 59% of re-treated pts with sALCL, as well as a manageable safety profile, including continued resolution or improvement of PN.
Keyword(s): CD30, Phase III, T cell lymphoma, Targeted therapy


