
Contributions
Abstract: EP503
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Elderly/unfit pts with DLBCL who are ineligible for standard first-line (1L) chemoimmunotherapy (CIT) due to frailty and comorbidities often receive either no treatment, reduced-dose R-CHOP, or therapies such as R-CVP and R-bendamustine: outcomes are often poor (Shewade, et al. ASH 2020). Mosun, a full-length, humanized, IgG1 CD20xCD3 bispecific antibody, has promising efficacy and tolerable safety in relapsed/refractory DLBCL (ongoing Phase I study NCT02500407; Schuster, et al. ASH 2019). Preliminary data from the Phase I/II, multicenter GO40554 study (NCT03677154) show notable efficacy and tolerability with Mosun monotherapy for elderly/unfit pts with 1L DLBCL who cannot tolerate full-dose CIT (Olszewski, et al. ASH 2020).
Aims
To describe updated clinical data from the GO40554 study.
Methods
Two safety-evaluation cohorts were assessed (Mosun 13.5mg and 30mg), followed by an expansion phase (Mosun 30mg). Pts were aged ≥80 years, or 60–79 years with impairment in ≥1 activity of daily living (ADL) or instrumental ADL, or impaired cardiac, renal or liver function precluding full-dose CIT. Pts received optional pre-treatment with prednisone (+/- vincristine), followed by intravenous Mosun in step-up doses on Days (D) 1 (1mg), 8 (2mg) and 15 (full dose: 13.5/30mg) of Cycle (C) 1 and full (C1D15) dose Mosun on D1 of each subsequent 21-day cycle. Pts with a complete response (CR) stop Mosun after 8 cycles; pts with a partial response (PR) or stable disease can continue Mosun for a total of 17 cycles.
Results
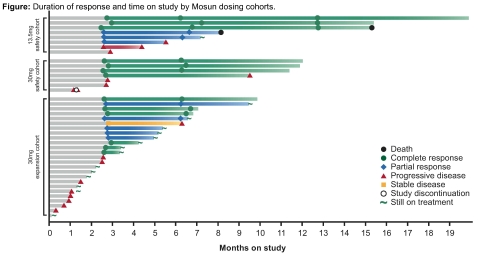
As of January 15, 2021, 40 pts had received Mosun (13.5mg safety cohort, n=8; 30mg safety cohort, n=7; 30mg expansion, n=25). Median age was 84 (67–100) years, 27 (67.5%) pts were female, 27 (67.5%) had ECOG performance status 0–1, 20 (50%) had Ann Arbor Stage III–IV and 32 (80%) had an IPI score ≥2. Pts received a median of 6 (range: 1–13) Mosun cycles; 2 pts in PR continued Mosun after 8 cycles. Of 40 pts, 35 (87.5%) had ≥1 adverse event (AE), 27 (67.5%) had ≥1 Mosun-related AE; 15 (37.5%) pts had a grade (Gr) 3–4 AE, 8 of which were Mosun-related. The most common (>10%) treatment-emergent AEs were cytokine release syndrome (CRS; n=9, 22.5%), abdominal pain (n=7, 17.5%), rash (n=5, 12.5%) and neutropenia (n=5, 12.5%); one pt had febrile neutropenia. Six (15%) pts had Gr 1 CRS per ASTCT (Lee, et al. 2019). Three (7.5%) pts had Gr 2 CRS events, all were managed with supportive care and fluids for hypotension, steroids and low-flow oxygen, as required (no tocilizumab, vasopressors, high-flow oxygen or intensive care required). One pt with Gr 1 CRS had Mosun-related Gr 2 neurotoxicity on C1D2 (inability to answer questions, drowsiness and weakness), which resolved after 2 days. One pt had a Gr 2 non-serious headache. No fatal AEs or Gr ≥3 neurologic AEs were observed. Between C1–C8, 14 (35%) pts discontinued Mosun due to progressive disease (PD); no pts discontinued due to AEs. For efficacy-evaluable pts (n=31), the overall response rate was 67.7% and CR rate was 41.9%. Responses after C8 (n [%]) with Mosun 13.5mg (N=8) were: CR: 3 (37.5); PR: 3 (37.5); PD: 2 (25); and after C4 or C8 with Mosun 30mg (N=23) were: CR: 10 (43.5); PR: 5 (21.7); PD: 3 (13). Of 13 pts with CR (11 ongoing), 4 durable responses have been observed ≥12 months from the start of therapy (Figure). Biomarker analyses are ongoing and will be provided.
Conclusion
Mosun continues to show promising efficacy, including durable responses, and tolerable safety for elderly/unfit pts with 1L DLBCL and should be further evaluated as a chemotherapy-free backbone for pts unable to tolerate standard CIT.
Keyword(s): Antibody, DLBCL, Elderly, Immunotherapy
Abstract: EP503
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Elderly/unfit pts with DLBCL who are ineligible for standard first-line (1L) chemoimmunotherapy (CIT) due to frailty and comorbidities often receive either no treatment, reduced-dose R-CHOP, or therapies such as R-CVP and R-bendamustine: outcomes are often poor (Shewade, et al. ASH 2020). Mosun, a full-length, humanized, IgG1 CD20xCD3 bispecific antibody, has promising efficacy and tolerable safety in relapsed/refractory DLBCL (ongoing Phase I study NCT02500407; Schuster, et al. ASH 2019). Preliminary data from the Phase I/II, multicenter GO40554 study (NCT03677154) show notable efficacy and tolerability with Mosun monotherapy for elderly/unfit pts with 1L DLBCL who cannot tolerate full-dose CIT (Olszewski, et al. ASH 2020).
Aims
To describe updated clinical data from the GO40554 study.
Methods
Two safety-evaluation cohorts were assessed (Mosun 13.5mg and 30mg), followed by an expansion phase (Mosun 30mg). Pts were aged ≥80 years, or 60–79 years with impairment in ≥1 activity of daily living (ADL) or instrumental ADL, or impaired cardiac, renal or liver function precluding full-dose CIT. Pts received optional pre-treatment with prednisone (+/- vincristine), followed by intravenous Mosun in step-up doses on Days (D) 1 (1mg), 8 (2mg) and 15 (full dose: 13.5/30mg) of Cycle (C) 1 and full (C1D15) dose Mosun on D1 of each subsequent 21-day cycle. Pts with a complete response (CR) stop Mosun after 8 cycles; pts with a partial response (PR) or stable disease can continue Mosun for a total of 17 cycles.
Results
As of January 15, 2021, 40 pts had received Mosun (13.5mg safety cohort, n=8; 30mg safety cohort, n=7; 30mg expansion, n=25). Median age was 84 (67–100) years, 27 (67.5%) pts were female, 27 (67.5%) had ECOG performance status 0–1, 20 (50%) had Ann Arbor Stage III–IV and 32 (80%) had an IPI score ≥2. Pts received a median of 6 (range: 1–13) Mosun cycles; 2 pts in PR continued Mosun after 8 cycles. Of 40 pts, 35 (87.5%) had ≥1 adverse event (AE), 27 (67.5%) had ≥1 Mosun-related AE; 15 (37.5%) pts had a grade (Gr) 3–4 AE, 8 of which were Mosun-related. The most common (>10%) treatment-emergent AEs were cytokine release syndrome (CRS; n=9, 22.5%), abdominal pain (n=7, 17.5%), rash (n=5, 12.5%) and neutropenia (n=5, 12.5%); one pt had febrile neutropenia. Six (15%) pts had Gr 1 CRS per ASTCT (Lee, et al. 2019). Three (7.5%) pts had Gr 2 CRS events, all were managed with supportive care and fluids for hypotension, steroids and low-flow oxygen, as required (no tocilizumab, vasopressors, high-flow oxygen or intensive care required). One pt with Gr 1 CRS had Mosun-related Gr 2 neurotoxicity on C1D2 (inability to answer questions, drowsiness and weakness), which resolved after 2 days. One pt had a Gr 2 non-serious headache. No fatal AEs or Gr ≥3 neurologic AEs were observed. Between C1–C8, 14 (35%) pts discontinued Mosun due to progressive disease (PD); no pts discontinued due to AEs. For efficacy-evaluable pts (n=31), the overall response rate was 67.7% and CR rate was 41.9%. Responses after C8 (n [%]) with Mosun 13.5mg (N=8) were: CR: 3 (37.5); PR: 3 (37.5); PD: 2 (25); and after C4 or C8 with Mosun 30mg (N=23) were: CR: 10 (43.5); PR: 5 (21.7); PD: 3 (13). Of 13 pts with CR (11 ongoing), 4 durable responses have been observed ≥12 months from the start of therapy (Figure). Biomarker analyses are ongoing and will be provided.

Conclusion
Mosun continues to show promising efficacy, including durable responses, and tolerable safety for elderly/unfit pts with 1L DLBCL and should be further evaluated as a chemotherapy-free backbone for pts unable to tolerate standard CIT.
Keyword(s): Antibody, DLBCL, Elderly, Immunotherapy


