
Contributions
Abstract: EP499
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Epcoritamab is a CD20xCD3 bispecific antibody that induces T-cell–mediated killing of CD20–positive malignant B-cells.
Aims
To present updated data, including progression-free survival (PFS) from the dose escalation part of the first-in-human phase 1/2 study of epcoritamab in patients with relapsed or refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL; NCT03625037).
Methods
Adults with R/R CD20+ B-NHL received flat-dose 1 mL SC epcoritamab (step-up dosing approach) in 28-day cycles (q1w: cycles 1–2; q2w: cycles 3–6; q4w thereafter) until disease progression or unacceptable toxicity. Step-up dosing and standard prophylaxis were used to mitigate severity of cytokine release syndrome (CRS). Informed consent was obtained from all patients prior to trial enrollment.
Results
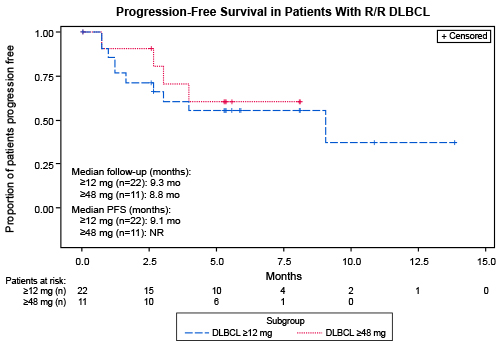
At data cut off (31 January 2021), 68 patients with B-NHL were enrolled across histologies including diffuse large B-cell lymphoma (DLBCL; n=46 [67.6%]; de novo and transformed), follicular lymphoma (FL; 12 [17.6%]), mantle cell lymphoma (MCL; 4 [5.9%]), and others (6 [8.8%]). The majority of patients were heavily pretreated (median [range] prior lines: DLBCL, 3 [1–6]; FL, 4.5 [1–18]); including prior CAR-T (n=6) and prior ASCT (n=10). At median follow-up of 14.1 months (DLBCL, 10.2 months; FL, 15.2 months), treatment was ongoing in 15 (22%) patients. The most common treatment-emergent adverse events (AEs) were pyrexia (69%), CRS (59%), and injection site reaction (47%). CRS events were all grade 1 or 2 and most occurred in cycle 1; neurotoxicity was limited (6%; grade 1: 3%; grade 3: 3%; all transient). One case of tumor lysis syndrome was observed (1.5%; grade 3); there were no cases of febrile neutropenia or treatment-related death. Overall response rates (ORRs) for patients with DLBCL at doses ≥12 mg (n=22) and ≥48 mg (n=11) were 68% (CR=46%; PR=23%) and 91% (CR=55%; PR=36%), respectively. The ORR for patients with FL at doses ≥12 mg (n=5) was 80% (CR=60%; PR=20%); the ORR for patients with MCL at doses ≥0.76 mg (n=4) was 50% (CR=25%; PR=25%). Responses deepened over time (PR converted to CR: DLBCL, 6 patients; FL, 3 patients). For patients with DLBCL, median (range) time to response was 1.4 (1.0–4.0) months; median (range) time to CR was 2.7 (1.1–3.9) months. For patients with FL, median (range) time to response was 1.9 (1.0–4.0) months. Among DLBCL patients achieving CR with doses ≥6 mg (n=11), none relapsed while on treatment. The median PFS for patients with DLBCL ≥12 mg (n=22) was 9.1 months (95% CI: 1.6, NE; median follow-up 9.3 months) and for patients with DLBCL ≥48 mg (n=11) median PFS was not reached (median follow-up 8.8 months) (Figure). Updated analyses will be presented.
Conclusion
With longer follow-up, SC epcoritamab demonstrated substantial single-agent activity, inducing deep and durable clinically meaningful responses, with a consistent safety profile. Notably no severe (grade ≥3) CRS events, no febrile neutropenia, and limited neurotoxicity was observed.
Keyword(s): Diffuse large B cell lymphoma, Hematological malignancy, Non-Hodgkin's lymphoma
Abstract: EP499
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Epcoritamab is a CD20xCD3 bispecific antibody that induces T-cell–mediated killing of CD20–positive malignant B-cells.
Aims
To present updated data, including progression-free survival (PFS) from the dose escalation part of the first-in-human phase 1/2 study of epcoritamab in patients with relapsed or refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL; NCT03625037).
Methods
Adults with R/R CD20+ B-NHL received flat-dose 1 mL SC epcoritamab (step-up dosing approach) in 28-day cycles (q1w: cycles 1–2; q2w: cycles 3–6; q4w thereafter) until disease progression or unacceptable toxicity. Step-up dosing and standard prophylaxis were used to mitigate severity of cytokine release syndrome (CRS). Informed consent was obtained from all patients prior to trial enrollment.
Results
At data cut off (31 January 2021), 68 patients with B-NHL were enrolled across histologies including diffuse large B-cell lymphoma (DLBCL; n=46 [67.6%]; de novo and transformed), follicular lymphoma (FL; 12 [17.6%]), mantle cell lymphoma (MCL; 4 [5.9%]), and others (6 [8.8%]). The majority of patients were heavily pretreated (median [range] prior lines: DLBCL, 3 [1–6]; FL, 4.5 [1–18]); including prior CAR-T (n=6) and prior ASCT (n=10). At median follow-up of 14.1 months (DLBCL, 10.2 months; FL, 15.2 months), treatment was ongoing in 15 (22%) patients. The most common treatment-emergent adverse events (AEs) were pyrexia (69%), CRS (59%), and injection site reaction (47%). CRS events were all grade 1 or 2 and most occurred in cycle 1; neurotoxicity was limited (6%; grade 1: 3%; grade 3: 3%; all transient). One case of tumor lysis syndrome was observed (1.5%; grade 3); there were no cases of febrile neutropenia or treatment-related death. Overall response rates (ORRs) for patients with DLBCL at doses ≥12 mg (n=22) and ≥48 mg (n=11) were 68% (CR=46%; PR=23%) and 91% (CR=55%; PR=36%), respectively. The ORR for patients with FL at doses ≥12 mg (n=5) was 80% (CR=60%; PR=20%); the ORR for patients with MCL at doses ≥0.76 mg (n=4) was 50% (CR=25%; PR=25%). Responses deepened over time (PR converted to CR: DLBCL, 6 patients; FL, 3 patients). For patients with DLBCL, median (range) time to response was 1.4 (1.0–4.0) months; median (range) time to CR was 2.7 (1.1–3.9) months. For patients with FL, median (range) time to response was 1.9 (1.0–4.0) months. Among DLBCL patients achieving CR with doses ≥6 mg (n=11), none relapsed while on treatment. The median PFS for patients with DLBCL ≥12 mg (n=22) was 9.1 months (95% CI: 1.6, NE; median follow-up 9.3 months) and for patients with DLBCL ≥48 mg (n=11) median PFS was not reached (median follow-up 8.8 months) (Figure). Updated analyses will be presented.

Conclusion
With longer follow-up, SC epcoritamab demonstrated substantial single-agent activity, inducing deep and durable clinically meaningful responses, with a consistent safety profile. Notably no severe (grade ≥3) CRS events, no febrile neutropenia, and limited neurotoxicity was observed.
Keyword(s): Diffuse large B cell lymphoma, Hematological malignancy, Non-Hodgkin's lymphoma


