
Contributions
Abstract: EP498
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Relapsed/refractory (r/r) lymphoma patients with comorbidities and/or advanced age are increasingly considered for treatment with CD19 CAR-T. However, there are no clear criteria to define fitness and limited data on the clinical benefit of CAR-T in the less fit patient population.
Aims
To analyse the outcomes of lymphoma patients approved for licenced CD19 CAR-T in the UK who were deemed unfit for autologous stem cell transplant (ASCT).
Methods
We analysed 400 consecutive r/r high-grade lymphoma patients approved for treatment with axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) between Dec 2018 and Nov 2020 across 10 UK centres. Data on patients’ fitness and comorbidities were retrospectively collected. Fitness for ASCT was defined by the treating team based on physical suitability irrespective of disease status.
Results
80/400 (20%) of patients approved for CAR-T were deemed unfit for ASCT (40 for age alone, 10 frailty, 11 cardiac function, 6 kidney function, 13 other/multiple comorbidities).
52/80 (65%) of patients in the ASCT-unfit cohort underwent CAR-T infusion; Reasons for not proceeding with treatment were as follows: 20 progressive disease (PD), 2 manufacturing failures, 1 cardiac failure, 1 diagnosis of dementia, 3 deaths. The drop-out rate from approval to infusion in the ASCT-unfit vs. ASCT-fit group was 34% vs. 24% (p=0.062).
27/52 (52%) patients received tisa-cel, 25 (48%) axi-cel (vs. 20% tisa-cel in fit patients; p‹0.0001). Patients’ median age was 71 years (range 47-78) vs. 56 years (range 18-72) in the ASCT-fit cohort (p‹0.0001). ASCT-unfit patients were more likely to have ECOG PS 1 at baseline (65% vs. 46%; p=0.02), high-risk IPI (24% vs. 9%; p=0.005), HCT-CI comorbidity score of 3+ (17% vs. 5%; p=0.005), lower GFR (‹50ml/min: 25% vs. 5%; p‹0.0001), and lower LVEF (‹50%: 18% vs. 6%; p=0.01). No other significant difference in baseline characteristics was observed.
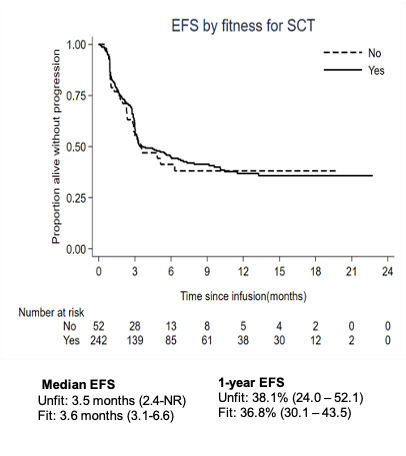
The ongoing 6-months ORR was 31% (all CR). With a median follow-up of 10.5 months, the median EFS/OS were 3.5/10.6 months and the 12-months EFS/OS 38/42%. There was no significant difference in efficacy outcomes between the ASCT-unfit and -fit cohorts. Moreover, no evidence of a difference between groups who were unfit due to age or comorbidities, nor evidence of worse prognosis for patients with impaired kidney- or cardiac function or high HCT-CI.
Among 52 ASCT-unfit patients, there was one case with G3 CRS (2%), and 6 patients (11%) with G3+ ICANS. No significant difference was observed in the incidence of any grade/G3+ CRS or ICANS, the use of tocilizumab or steroids, ICU admission rate, and the incidence of G3+ cytopenias post CAR-T compared to the ASCT-fit cohort.
Conclusion
Results from our large, prospective real-world cohort demonstrate that CD19 CAR-T can be safely delivered in carefully selected older patients and patients with comorbidities who are not deemed suitable for transplant. There was a trend towards a higher drop-out rate in less fit patients, but patients who successfully undergo infusion appear to have the same long-term benefit from CD19 CAR-T as ASCT-fit patients and should be considered for this potentially curative treatment.
Keyword(s):
Abstract: EP498
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Relapsed/refractory (r/r) lymphoma patients with comorbidities and/or advanced age are increasingly considered for treatment with CD19 CAR-T. However, there are no clear criteria to define fitness and limited data on the clinical benefit of CAR-T in the less fit patient population.
Aims
To analyse the outcomes of lymphoma patients approved for licenced CD19 CAR-T in the UK who were deemed unfit for autologous stem cell transplant (ASCT).
Methods
We analysed 400 consecutive r/r high-grade lymphoma patients approved for treatment with axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) between Dec 2018 and Nov 2020 across 10 UK centres. Data on patients’ fitness and comorbidities were retrospectively collected. Fitness for ASCT was defined by the treating team based on physical suitability irrespective of disease status.
Results
80/400 (20%) of patients approved for CAR-T were deemed unfit for ASCT (40 for age alone, 10 frailty, 11 cardiac function, 6 kidney function, 13 other/multiple comorbidities).
52/80 (65%) of patients in the ASCT-unfit cohort underwent CAR-T infusion; Reasons for not proceeding with treatment were as follows: 20 progressive disease (PD), 2 manufacturing failures, 1 cardiac failure, 1 diagnosis of dementia, 3 deaths. The drop-out rate from approval to infusion in the ASCT-unfit vs. ASCT-fit group was 34% vs. 24% (p=0.062).
27/52 (52%) patients received tisa-cel, 25 (48%) axi-cel (vs. 20% tisa-cel in fit patients; p‹0.0001). Patients’ median age was 71 years (range 47-78) vs. 56 years (range 18-72) in the ASCT-fit cohort (p‹0.0001). ASCT-unfit patients were more likely to have ECOG PS 1 at baseline (65% vs. 46%; p=0.02), high-risk IPI (24% vs. 9%; p=0.005), HCT-CI comorbidity score of 3+ (17% vs. 5%; p=0.005), lower GFR (‹50ml/min: 25% vs. 5%; p‹0.0001), and lower LVEF (‹50%: 18% vs. 6%; p=0.01). No other significant difference in baseline characteristics was observed.
The ongoing 6-months ORR was 31% (all CR). With a median follow-up of 10.5 months, the median EFS/OS were 3.5/10.6 months and the 12-months EFS/OS 38/42%. There was no significant difference in efficacy outcomes between the ASCT-unfit and -fit cohorts. Moreover, no evidence of a difference between groups who were unfit due to age or comorbidities, nor evidence of worse prognosis for patients with impaired kidney- or cardiac function or high HCT-CI.
Among 52 ASCT-unfit patients, there was one case with G3 CRS (2%), and 6 patients (11%) with G3+ ICANS. No significant difference was observed in the incidence of any grade/G3+ CRS or ICANS, the use of tocilizumab or steroids, ICU admission rate, and the incidence of G3+ cytopenias post CAR-T compared to the ASCT-fit cohort.

Conclusion
Results from our large, prospective real-world cohort demonstrate that CD19 CAR-T can be safely delivered in carefully selected older patients and patients with comorbidities who are not deemed suitable for transplant. There was a trend towards a higher drop-out rate in less fit patients, but patients who successfully undergo infusion appear to have the same long-term benefit from CD19 CAR-T as ASCT-fit patients and should be considered for this potentially curative treatment.
Keyword(s):


