
Contributions
Abstract: EP496
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Tafasitamab is a humanized, Fc-modified anti-CD19 monoclonal antibody that enhances antibody‑dependent cellular cytotoxicity and phagocytosis. It is FDA-approved in combination with lenalidomide for adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), including arising from low‑grade lymphoma, who are ineligible for autologous stem cell transplant (ASCT).
Aims
First-MIND (NCT04134936) is a Phase Ib, open‑label, randomized study to assess the safety and preliminary efficacy of tafasitamab + R-CHOP or tafasitamab + lenalidomide + R‑CHOP in patients with newly diagnosed DLBCL.
Methods
Eligible patients were aged ≥18 years, treatment-naïve, with DLBCL, international prognostic index (IPI) 2–5 and Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2. Patients with known double- or triple-hit and transformed lymphoma were excluded. Treatment comprised six 21‑day cycles of R-CHOP + tafasitamab (12 mg/kg IV, Day [D] 1, 8 and 15) (arm A) or R-CHOP + tafasitamab (12 mg/kg IV, D1, 8 and 15) + lenalidomide (25 mg orally, D1–10) (arm B). G-CSF and VTE prophylaxis was mandatory. Primary objective is safety; secondary objectives include overall response rate (ORR) and PET-CR rate at end of treatment, progression-free survival, long‑term safety, pharmacokinetics and immunogenicity.
Results
From Dec 2019 to Aug 2020, 83 patients were screened in nine countries across Europe and the US; 66 were randomized (arm A: n=33; arm B, n=33). Data cut-off: 9 Dec 2020; the study is ongoing. Median age was 64.5 years (range 20–86 years). Overall, 30% of patients (20/66) were ≥70 years and many had high-risk disease: IPI 2 28.8%, IPI 3 45.5%, IPI 4 25.8%. ECOG PS: 47.0% of patients were ECOG PS 0, 43.9% PS 1, 9.1% PS 2. Most patients had stage III/IV disease (92%); 45.5% had bulky disease.
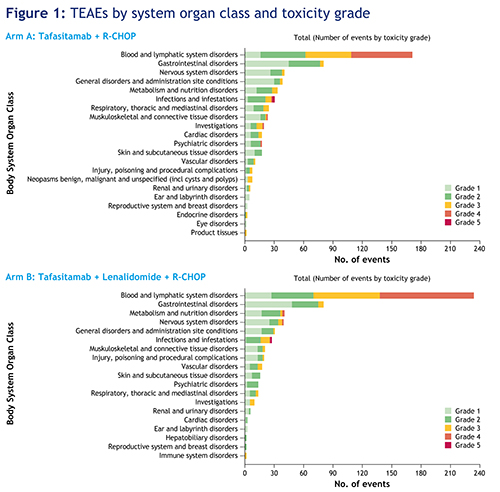
All patients experienced a treatment-emergent adverse event (TEAE); 555 events occurred in arm A and 570 in arm B. Grade ≥3 neutropenia was observed in 54.5% (arm A) and 66.7% (arm B) of patients. Grade ≥3 thrombocytopenia was observed in 12.1% (arm A) and 30.3% (arm B) of patients. Six patients in each arm experienced febrile neutropenia. Diarrhea, fatigue and vomiting were comparable between the two arms. Grade ≥3 nervous system disorders were experienced by 6.1% of patients in arm A and 12.1% in arm B (the majority of events were polyneuropathies related to vincristine). Infusion-related reactions to both rituximab and tafasitamab occurred in 12.1% (arm A) and 18.2% (arm B) of patients, and 21.2% (arm A) and 27.3% (arm B) of patients had a grade ≥3 infection and/or infestation (TEAEs by system organ class [SOC] shown in Figure 1). Serious TEAEs occurred in 42.4% (arm A) and 51.5% (arm B) of patients. There were three deaths unrelated to tafasitamab and/or lenalidomide (sepsis, urosepsis and COVID-19 pneumonia). Dose intensity of R‑CHOP was maintained in both treatment arms.
Among 60 patients who completed tumor assessments after cycle 3, ORR was 89.7% (arm A) and 93.5% (arm B).
Conclusion
These data suggest that R-CHOP + tafasitamab or tafasitamab + lenalidomide is tolerable in patients with treatment-naïve DLBCL. Dosing and scheduling of R-CHOP is not affected by the addition of tafasitamab. Toxicities are similar to those expected with R-CHOP alone or R-CHOP + lenalidomide. Updated safety and early efficacy data will be presented at the conference.
Keyword(s): CD19, DLBCL, Lymphoma, Monoclonal antibody
Abstract: EP496
Type: E-Poster Presentation
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Tafasitamab is a humanized, Fc-modified anti-CD19 monoclonal antibody that enhances antibody‑dependent cellular cytotoxicity and phagocytosis. It is FDA-approved in combination with lenalidomide for adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), including arising from low‑grade lymphoma, who are ineligible for autologous stem cell transplant (ASCT).
Aims
First-MIND (NCT04134936) is a Phase Ib, open‑label, randomized study to assess the safety and preliminary efficacy of tafasitamab + R-CHOP or tafasitamab + lenalidomide + R‑CHOP in patients with newly diagnosed DLBCL.
Methods
Eligible patients were aged ≥18 years, treatment-naïve, with DLBCL, international prognostic index (IPI) 2–5 and Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2. Patients with known double- or triple-hit and transformed lymphoma were excluded. Treatment comprised six 21‑day cycles of R-CHOP + tafasitamab (12 mg/kg IV, Day [D] 1, 8 and 15) (arm A) or R-CHOP + tafasitamab (12 mg/kg IV, D1, 8 and 15) + lenalidomide (25 mg orally, D1–10) (arm B). G-CSF and VTE prophylaxis was mandatory. Primary objective is safety; secondary objectives include overall response rate (ORR) and PET-CR rate at end of treatment, progression-free survival, long‑term safety, pharmacokinetics and immunogenicity.
Results
From Dec 2019 to Aug 2020, 83 patients were screened in nine countries across Europe and the US; 66 were randomized (arm A: n=33; arm B, n=33). Data cut-off: 9 Dec 2020; the study is ongoing. Median age was 64.5 years (range 20–86 years). Overall, 30% of patients (20/66) were ≥70 years and many had high-risk disease: IPI 2 28.8%, IPI 3 45.5%, IPI 4 25.8%. ECOG PS: 47.0% of patients were ECOG PS 0, 43.9% PS 1, 9.1% PS 2. Most patients had stage III/IV disease (92%); 45.5% had bulky disease.
All patients experienced a treatment-emergent adverse event (TEAE); 555 events occurred in arm A and 570 in arm B. Grade ≥3 neutropenia was observed in 54.5% (arm A) and 66.7% (arm B) of patients. Grade ≥3 thrombocytopenia was observed in 12.1% (arm A) and 30.3% (arm B) of patients. Six patients in each arm experienced febrile neutropenia. Diarrhea, fatigue and vomiting were comparable between the two arms. Grade ≥3 nervous system disorders were experienced by 6.1% of patients in arm A and 12.1% in arm B (the majority of events were polyneuropathies related to vincristine). Infusion-related reactions to both rituximab and tafasitamab occurred in 12.1% (arm A) and 18.2% (arm B) of patients, and 21.2% (arm A) and 27.3% (arm B) of patients had a grade ≥3 infection and/or infestation (TEAEs by system organ class [SOC] shown in Figure 1). Serious TEAEs occurred in 42.4% (arm A) and 51.5% (arm B) of patients. There were three deaths unrelated to tafasitamab and/or lenalidomide (sepsis, urosepsis and COVID-19 pneumonia). Dose intensity of R‑CHOP was maintained in both treatment arms.
Among 60 patients who completed tumor assessments after cycle 3, ORR was 89.7% (arm A) and 93.5% (arm B).

Conclusion
These data suggest that R-CHOP + tafasitamab or tafasitamab + lenalidomide is tolerable in patients with treatment-naïve DLBCL. Dosing and scheduling of R-CHOP is not affected by the addition of tafasitamab. Toxicities are similar to those expected with R-CHOP alone or R-CHOP + lenalidomide. Updated safety and early efficacy data will be presented at the conference.
Keyword(s): CD19, DLBCL, Lymphoma, Monoclonal antibody


