
Contributions
Abstract: EP487
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Patients (pts) with standard-risk acute myeloid leukemia (AML) are not regularly planned for up-front allogeneic stem cell transplantation (allo-SCT). Yet, some of these pts fail reaching minimal residual disease (MRD) negativity with standard chemotherapy and are allocated to allo-SCT during 1st complete remission. Gemtuzumab ozogamicin (GO), an antibody-toxin conjugate targeting CD33+ cells, is associated with overall survival (OS) benefit and reduced relapse rate in standard-risk AML. The FDA approval of GO use as an addition to intensive chemotherapy relies on results of the French ALFA-0701 trial where pts were assigned to receive GO (3mg/m2 on days 1,4,7) combined with the 7+3 regimen as induction and combined with daunorubicin/cytarabine (GO: 3mg/m2 on day 1) for 2 courses as consolidation. However, in many countries, reimbursement issues preclude the use of GO as part of induction.
Aims
The Israeli Leukemia Group recommended including GO (capped at 4.5mg total on day 1) in any consolidation therapy comprising high-dose cytarabine (HIDAC) for standard-risk AML pts, even if GO was not applied as part of induction. The present study aimed to evaluate outcomes of pts who received GO+HIDAC as consolidation, in terms of MRD clearance and toxicities.
Methods
Electronic medical records of 4 Israeli tertiary care centers were retrospectively reviewed to identify standard-risk AML pts treated with 7+3 induction and HIDAC consolidation. Pts were then grouped into two cohorts based on GO use in combination with HIDAC (GO vs No-GO). The cohorts were compared in terms of OS, progression-free survival (PFS) and MRD clearance after 2 intensive chemotherapy cycles. For toxicity evaluation, the number of infectious events, blood products used, hemorrhage events, mucositis (≥grade 3) events, veno-occlusive disease (VOD) events and occurrence of prolonged thrombocytopenia (platelet count <100 at 30 days post-treatment) were analyzed. The dose intensity per unit time was also assessed. Of note, hospitalization duration was not included in the final analysis due to inter-hospital admission policy differences.
Results
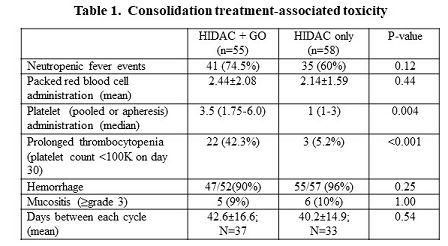
The GO and No-GO cohorts included 22 and 21 pts, respectively. They were comparable in terms of age, sex and specific driver mutations other than the combination of NPM1+ low allelic ratio-FLT3-ITD that was less prevalent in the GO cohort (5% vs 27%; p=0.046). The majority of pts (77% vs 57%; p=0.2) received intensified induction (daunorubicin 90mg/m2 or its equal). Among the pts whose MRD data were available, MRD clearance was reached in all those who received GO and only in two thirds of the No-GO cohort (12/12 vs 7/11; p=0.037). No VOD or treatment-related death occurred. A total of 113 consolidation cycles were given: 55 HIDAC+GO and 58 HIDAC cycles (Table 1). The GO+HIDAC regimen was associated with higher rates of platelet transfusion (median 3.5 vs 1 of pooled or apheresis platelets) and prolonged thrombocytopenia (42.3% vs 5.2%; p<0.001). Only 7 bleeding events occurred (5 after GO injection). No life threatening bleeds were recorded. GO administration did not entail higher neutropenic fever rates (74.5% vs 60%; p=0.12), excessive use of packed cell units (p=0.44), treatment delays or therapy-related death.
Conclusion
While associated with high bleeding risk attributed to severe and prolonged thrombocytopenia, consolidation with HIDAC+GO is safe and provides high rates of MRD eradication. Standard-risk AML patients who do not receive GO in induction may benefit from its addition to consolidation regimens.
Keyword(s): Acute myeloid leukemia, Gemtuzumab ozogamicin, Minimal residual disease (MRD)
Abstract: EP487
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Patients (pts) with standard-risk acute myeloid leukemia (AML) are not regularly planned for up-front allogeneic stem cell transplantation (allo-SCT). Yet, some of these pts fail reaching minimal residual disease (MRD) negativity with standard chemotherapy and are allocated to allo-SCT during 1st complete remission. Gemtuzumab ozogamicin (GO), an antibody-toxin conjugate targeting CD33+ cells, is associated with overall survival (OS) benefit and reduced relapse rate in standard-risk AML. The FDA approval of GO use as an addition to intensive chemotherapy relies on results of the French ALFA-0701 trial where pts were assigned to receive GO (3mg/m2 on days 1,4,7) combined with the 7+3 regimen as induction and combined with daunorubicin/cytarabine (GO: 3mg/m2 on day 1) for 2 courses as consolidation. However, in many countries, reimbursement issues preclude the use of GO as part of induction.
Aims
The Israeli Leukemia Group recommended including GO (capped at 4.5mg total on day 1) in any consolidation therapy comprising high-dose cytarabine (HIDAC) for standard-risk AML pts, even if GO was not applied as part of induction. The present study aimed to evaluate outcomes of pts who received GO+HIDAC as consolidation, in terms of MRD clearance and toxicities.
Methods
Electronic medical records of 4 Israeli tertiary care centers were retrospectively reviewed to identify standard-risk AML pts treated with 7+3 induction and HIDAC consolidation. Pts were then grouped into two cohorts based on GO use in combination with HIDAC (GO vs No-GO). The cohorts were compared in terms of OS, progression-free survival (PFS) and MRD clearance after 2 intensive chemotherapy cycles. For toxicity evaluation, the number of infectious events, blood products used, hemorrhage events, mucositis (≥grade 3) events, veno-occlusive disease (VOD) events and occurrence of prolonged thrombocytopenia (platelet count <100 at 30 days post-treatment) were analyzed. The dose intensity per unit time was also assessed. Of note, hospitalization duration was not included in the final analysis due to inter-hospital admission policy differences.
Results
The GO and No-GO cohorts included 22 and 21 pts, respectively. They were comparable in terms of age, sex and specific driver mutations other than the combination of NPM1+ low allelic ratio-FLT3-ITD that was less prevalent in the GO cohort (5% vs 27%; p=0.046). The majority of pts (77% vs 57%; p=0.2) received intensified induction (daunorubicin 90mg/m2 or its equal). Among the pts whose MRD data were available, MRD clearance was reached in all those who received GO and only in two thirds of the No-GO cohort (12/12 vs 7/11; p=0.037). No VOD or treatment-related death occurred. A total of 113 consolidation cycles were given: 55 HIDAC+GO and 58 HIDAC cycles (Table 1). The GO+HIDAC regimen was associated with higher rates of platelet transfusion (median 3.5 vs 1 of pooled or apheresis platelets) and prolonged thrombocytopenia (42.3% vs 5.2%; p<0.001). Only 7 bleeding events occurred (5 after GO injection). No life threatening bleeds were recorded. GO administration did not entail higher neutropenic fever rates (74.5% vs 60%; p=0.12), excessive use of packed cell units (p=0.44), treatment delays or therapy-related death.

Conclusion
While associated with high bleeding risk attributed to severe and prolonged thrombocytopenia, consolidation with HIDAC+GO is safe and provides high rates of MRD eradication. Standard-risk AML patients who do not receive GO in induction may benefit from its addition to consolidation regimens.
Keyword(s): Acute myeloid leukemia, Gemtuzumab ozogamicin, Minimal residual disease (MRD)


