
Contributions
Abstract: EP484
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
About 25% of newly diagnosed adult AML patients(pts) with normal cytogenetics have Fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD). And it confers unfavorable prognosis, however, factors associated with clinical outcomes of FLT3-ITD+ mutations are still controversial.
Aims
To find out the variables associated with the different outcomes of ITD+ patients(pts).
Methods
We evaluated 106 adult AML pts with this genotype who were diagnosed at Peking University People’s Hospital and achieved histological complete remission within two cycles of induction therapy. And we analysis the status of mutations for all pts based on next-generation sequencing.
Results
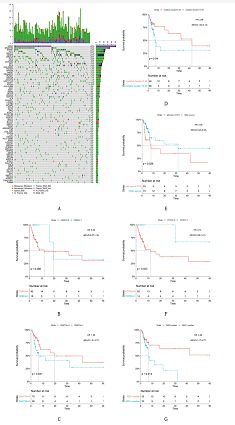
In our cohort, we totally found 171 ITDs(1 ITD: 60 pts, 2 ITDs: 31 pts, 3 ITDs: 11 pts and 4 ITDs: 4 pts). Of the 171 ITDs, 102(59.6%) located in the juxtamembrane domain(JMD); 24(14.0%) located in the tyrosine kinase domain-1(TKD1); 25(14.6%) located in the hinge region(HR) and 20 (18.7%) located in the other region of FLT3 receptor. Median of insert lengths was 65 bp[range,18-259 bp], median of variant allele frequency (VAF) was 0.45[range,0.04-0.78]. We founded that a better prognosis could be noticed if the inserted sequence is derived from wild type FLT3 gene fragment sequence[p=0.026 for leukemia-free survival (LFS) with transplant censored, p=0.039 for overall survival (OS) with transplant censored].We also focused on the co-mutated genes of FLT3-ITD and noticed pts with CEBPAbi-mut[n=16, p=0.066 for LFS, p=0.092 for OS],and POTEH[n=14, p=0.023 for LFS, p=0.2 for OS] showed an advantage in survival. However, high mutation burden[VAF> 0.45(established by using the ROC curve for maximum LFS), p=0.04 for LFS, p=0.077 for (OS)]; the mutation of DNMT3A [n=36, p=0.041 for LFS, p=0.13 for OS]; high WBC[> 29.83*10^9cells/L(median), p=0.014 for LFS, p=0.015 for OS] predicted worse survival. In addition, there were no obvious correlation of the insert site, the lengths of ITDs and the insert ITD number with the unfavorable prognosis of FLT3-ITD+ pts.
Conclusion
In this study, we revealed that POTEHmut, CEBPAbi-mut and ITDs which were derived from wild type FLT3 gene fragment always had a better survival, however, DNMT3Amut, high mutation burden and high WBC had a worse survival. Our study was beneficial to in-depth understanding of the FLT3-ITD’s characteristics and the genes combined with it. It also had guiding significance for clinical risk stratification of FLT3-ITD+ AML patients with normal cytogenetics.
Keyword(s): Flt3-ITD
Abstract: EP484
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
About 25% of newly diagnosed adult AML patients(pts) with normal cytogenetics have Fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD). And it confers unfavorable prognosis, however, factors associated with clinical outcomes of FLT3-ITD+ mutations are still controversial.
Aims
To find out the variables associated with the different outcomes of ITD+ patients(pts).
Methods
We evaluated 106 adult AML pts with this genotype who were diagnosed at Peking University People’s Hospital and achieved histological complete remission within two cycles of induction therapy. And we analysis the status of mutations for all pts based on next-generation sequencing.
Results
In our cohort, we totally found 171 ITDs(1 ITD: 60 pts, 2 ITDs: 31 pts, 3 ITDs: 11 pts and 4 ITDs: 4 pts). Of the 171 ITDs, 102(59.6%) located in the juxtamembrane domain(JMD); 24(14.0%) located in the tyrosine kinase domain-1(TKD1); 25(14.6%) located in the hinge region(HR) and 20 (18.7%) located in the other region of FLT3 receptor. Median of insert lengths was 65 bp[range,18-259 bp], median of variant allele frequency (VAF) was 0.45[range,0.04-0.78]. We founded that a better prognosis could be noticed if the inserted sequence is derived from wild type FLT3 gene fragment sequence[p=0.026 for leukemia-free survival (LFS) with transplant censored, p=0.039 for overall survival (OS) with transplant censored].We also focused on the co-mutated genes of FLT3-ITD and noticed pts with CEBPAbi-mut[n=16, p=0.066 for LFS, p=0.092 for OS],and POTEH[n=14, p=0.023 for LFS, p=0.2 for OS] showed an advantage in survival. However, high mutation burden[VAF> 0.45(established by using the ROC curve for maximum LFS), p=0.04 for LFS, p=0.077 for (OS)]; the mutation of DNMT3A [n=36, p=0.041 for LFS, p=0.13 for OS]; high WBC[> 29.83*10^9cells/L(median), p=0.014 for LFS, p=0.015 for OS] predicted worse survival. In addition, there were no obvious correlation of the insert site, the lengths of ITDs and the insert ITD number with the unfavorable prognosis of FLT3-ITD+ pts.

Conclusion
In this study, we revealed that POTEHmut, CEBPAbi-mut and ITDs which were derived from wild type FLT3 gene fragment always had a better survival, however, DNMT3Amut, high mutation burden and high WBC had a worse survival. Our study was beneficial to in-depth understanding of the FLT3-ITD’s characteristics and the genes combined with it. It also had guiding significance for clinical risk stratification of FLT3-ITD+ AML patients with normal cytogenetics.
Keyword(s): Flt3-ITD


