
Contributions
Abstract: EP482
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
High-dose cytarabine is currently a well accepted post remission therapy for younger patients with acute myeloid leukemia (AML) in first complete remission (CR1). No standard exists for older (≥ 60 years) patients. Although intermediate-dose cytarabine (IDAC) delivered every 12 hours for three days is regularly used in older fit patients, several academic groups also apply an outpatient schedule by standard dose cytarabine associated with single-dose anthracycline in monthly courses (SDAC).
Aims
To compare outcome of elderly patients with AML in CR1 receiving post-remission therapy, either with SDAC and anthracycline or with IDAC as single agent
Methods
Patients ≥ 60 years-old with non-M3 AML in CR1 after intensive chemotherapy included in French DATAML registry (Bordeaux and Toulouse) between 2007 and 2017 were retrospectively reviewed for study specific data collection. Patients had to receive at least one course of post-remission chemotherapy, either with SDAC or with IDAC. SDAC regimen consisted of six to seven 28-days courses of idarubicine (8mg/m2) D1 plus cytarabine (50mg/m2 BID in sub-cutaneous injection) D1-D5. IDAC regimen consisted of three 35-days course of cytarabine (1,5g/m2 in 2 hours infusion) every 12h for a total of 6 injections. Allogeneic stem cell transplantation (alloSCT) was performed at the physician’s discretion.
Results
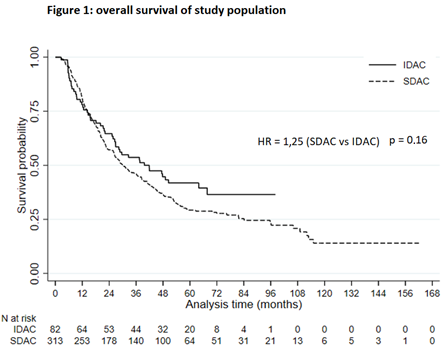
Population: 395 patients were included. Median age was 68 years. 82 patients received IDAC regimen and 313 received SDAC regimen. IDAC patients were younger (median age 65 versus 69, p <0.001), but have more adverse cytogenetics risk (31% vs 11%, p < 0.001) and were more likely secondary (35% vs 18%) or therapy related (18% vs 3%) (p < 0.001). Median course of post-remission therapy was 3 for IDAC and 5 for SDAC regimen. AlloSCT has been performed in 62 patients. Outcome: After a median follow up of 72 months, median OS was 40 months for IDAC vs 30 months for SDAC patients (p = 0.16), and median LFS was 21 months vs 17.5 months, respectively (p = 0.187). In multivariate analysis, factors associated with better OS were only Charlsons comobirdity index < 2, favorable ELN2010 category and alloSCT in CR1 (with an HR = 0.5). Post remission schedule was not independently associated to outcome. Tolerance: 1 year non relapse mortality was 10% for the whole cohort, without statistical difference between IDAC and SDAC patients. Deaths in remission during treatment were unfrequent (< 5%) in both groups. The rate of infectious events needing parenteral antibiotic was significantly lower with SDAC compared to IDAC (36.1% vs 74.4%) (p < 0.01) as well as the rate of documented bacteremia (15.7% vs 41.5%, p < 0.001). Median red blood cell transfusion requirement per cure was 1 (IQR 0,3 – 2) for SDAC vs 3 (IQR 2 – 4) for IDAC, and median platelet transfusion requirement per cure was 0,8 (IQR 0,2 – 1,4) vs 2,5 (IQR 1,6 – 3,3), respectively (p < 0.001 for both). Duration of hospitalization for the whole post-remission program was shorter with SDAC (12 days, IQR 7 – 19) compared to IDAC (32.5 days, IQR 22.5 – 38.5) (p < 0.001).
Conclusion
Although IDAC is feasible in fit AML patients over 60, the outpatient SDAC regimen induced low myelosuppression, decreased rate of infections and transfusions, and therefore significantly reduced the length of hospital stay without affecting outcomes.
Keyword(s):
Abstract: EP482
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
High-dose cytarabine is currently a well accepted post remission therapy for younger patients with acute myeloid leukemia (AML) in first complete remission (CR1). No standard exists for older (≥ 60 years) patients. Although intermediate-dose cytarabine (IDAC) delivered every 12 hours for three days is regularly used in older fit patients, several academic groups also apply an outpatient schedule by standard dose cytarabine associated with single-dose anthracycline in monthly courses (SDAC).
Aims
To compare outcome of elderly patients with AML in CR1 receiving post-remission therapy, either with SDAC and anthracycline or with IDAC as single agent
Methods
Patients ≥ 60 years-old with non-M3 AML in CR1 after intensive chemotherapy included in French DATAML registry (Bordeaux and Toulouse) between 2007 and 2017 were retrospectively reviewed for study specific data collection. Patients had to receive at least one course of post-remission chemotherapy, either with SDAC or with IDAC. SDAC regimen consisted of six to seven 28-days courses of idarubicine (8mg/m2) D1 plus cytarabine (50mg/m2 BID in sub-cutaneous injection) D1-D5. IDAC regimen consisted of three 35-days course of cytarabine (1,5g/m2 in 2 hours infusion) every 12h for a total of 6 injections. Allogeneic stem cell transplantation (alloSCT) was performed at the physician’s discretion.
Results
Population: 395 patients were included. Median age was 68 years. 82 patients received IDAC regimen and 313 received SDAC regimen. IDAC patients were younger (median age 65 versus 69, p <0.001), but have more adverse cytogenetics risk (31% vs 11%, p < 0.001) and were more likely secondary (35% vs 18%) or therapy related (18% vs 3%) (p < 0.001). Median course of post-remission therapy was 3 for IDAC and 5 for SDAC regimen. AlloSCT has been performed in 62 patients. Outcome: After a median follow up of 72 months, median OS was 40 months for IDAC vs 30 months for SDAC patients (p = 0.16), and median LFS was 21 months vs 17.5 months, respectively (p = 0.187). In multivariate analysis, factors associated with better OS were only Charlsons comobirdity index < 2, favorable ELN2010 category and alloSCT in CR1 (with an HR = 0.5). Post remission schedule was not independently associated to outcome. Tolerance: 1 year non relapse mortality was 10% for the whole cohort, without statistical difference between IDAC and SDAC patients. Deaths in remission during treatment were unfrequent (< 5%) in both groups. The rate of infectious events needing parenteral antibiotic was significantly lower with SDAC compared to IDAC (36.1% vs 74.4%) (p < 0.01) as well as the rate of documented bacteremia (15.7% vs 41.5%, p < 0.001). Median red blood cell transfusion requirement per cure was 1 (IQR 0,3 – 2) for SDAC vs 3 (IQR 2 – 4) for IDAC, and median platelet transfusion requirement per cure was 0,8 (IQR 0,2 – 1,4) vs 2,5 (IQR 1,6 – 3,3), respectively (p < 0.001 for both). Duration of hospitalization for the whole post-remission program was shorter with SDAC (12 days, IQR 7 – 19) compared to IDAC (32.5 days, IQR 22.5 – 38.5) (p < 0.001).

Conclusion
Although IDAC is feasible in fit AML patients over 60, the outpatient SDAC regimen induced low myelosuppression, decreased rate of infections and transfusions, and therefore significantly reduced the length of hospital stay without affecting outcomes.
Keyword(s):


