Contributions
Abstract: EP479
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Enasidenib (ENA), an oral inhibitor of mutated IDH2 (IDH2mut), has recently been approved by the FDA in monotherapy based on a phase 2 clinical trial in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML) (Stein et al Blood 2019). To date, as far as we know there are no studies reporting enasidenib efficacy and safety outside of Clinical trial.
Aims
To analyze the characteristics of the population that has received this treatment and the effectiveness and tolerance of ENA used outside of clinical trial.
Methods
Multicenter retrospective study including patients with IDH2-mut AML and MDS treated in Spain (2017-2020) with ENA outside of Clinical trial (compassionate use).
Results
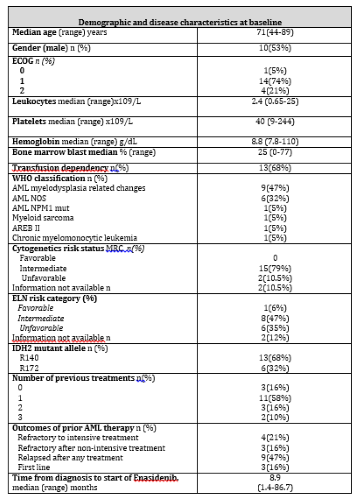
ENA was requested for 25 patients in 11 Spanish centers. Three patients died before starting ENA due to disease progression. Of the remaining 22, 3 did not complete the first course of ENA due to disease progression. We analyzed 19 patients for effectiveness and toxicity, 17 were AMLs, 1 AREB II and 1 CMML. Median age was 71 years (44-89) other baseline characteristics detailed in Table 1. The disease stage and treatment received prior to ENA were: 4 patients were refractory to intensive treatment (IT), 3 refractory after non-intensive treatment (NIT), 9 relapsed [5 after NIT and 4 after IT (3 of them post-allotransplant)] and 3 patients received ENA in the first line (1L). Most (11 patients) had received a previous line of treatment, 5 patients ≥ 2 lines.
The overall response rate (ORR) was 47% (N=9), 6 patients (31%) achieved complete response (CR). One patient performed a subsequent allogeneic transplant. The median number of cycles to best response was 3 (extremes 1-8). There were no differences in ORR according to disease or patient characteristics. Four (30%) of the 13 transfusion-dependent patients achieved transfusion independence.
With a median follow-up of 10.3 months (m) (3.4-19.8), median overall survival (OS) was 8.7m, with an OS at 1 year (1y-OS) of 47%±24%. Excluding the 3 patients treated in 1L there was no difference in OS depending on disease status/previous treatment. 1y-OS was significantly better in patients who achieved ORR (83%±30% vs 12%±22%; p<0.001). The median event-free survival (EFS) was 6.9 m (95%CI 2.01-11.78), being significantly better in the group that achieved ORR compared to the rest (1y-OS :74%±32% vs 10%±18%; p<0.001) and better in those who achieved CR (1y-OS: 100% vs 11%±20%; p=0.001). Remarkable the three patients treated after alloSCT are alive and disease free at time of reporting.
There were no treatment related deaths. Regarding specific toxicities of ENA 4(21%) cases presented leukocytosis, 3 (15%) differentiation syndrome, 6 (31%) hyperbilirubinemia. There were 3 admissions due to toxicity and in 2 patients ENA was definitively discontinued due to toxicity.
Conclusion
The results of this study confirm both the effectiveness and the good drug tolerability reported with ENA in the Phase II study
Funding: PI16/01027 and PI19/01476 (JE; MDB)
Keyword(s): AML, Enasidenib
Abstract: EP479
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Enasidenib (ENA), an oral inhibitor of mutated IDH2 (IDH2mut), has recently been approved by the FDA in monotherapy based on a phase 2 clinical trial in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML) (Stein et al Blood 2019). To date, as far as we know there are no studies reporting enasidenib efficacy and safety outside of Clinical trial.
Aims
To analyze the characteristics of the population that has received this treatment and the effectiveness and tolerance of ENA used outside of clinical trial.
Methods
Multicenter retrospective study including patients with IDH2-mut AML and MDS treated in Spain (2017-2020) with ENA outside of Clinical trial (compassionate use).
Results
ENA was requested for 25 patients in 11 Spanish centers. Three patients died before starting ENA due to disease progression. Of the remaining 22, 3 did not complete the first course of ENA due to disease progression. We analyzed 19 patients for effectiveness and toxicity, 17 were AMLs, 1 AREB II and 1 CMML. Median age was 71 years (44-89) other baseline characteristics detailed in Table 1. The disease stage and treatment received prior to ENA were: 4 patients were refractory to intensive treatment (IT), 3 refractory after non-intensive treatment (NIT), 9 relapsed [5 after NIT and 4 after IT (3 of them post-allotransplant)] and 3 patients received ENA in the first line (1L). Most (11 patients) had received a previous line of treatment, 5 patients ≥ 2 lines.
The overall response rate (ORR) was 47% (N=9), 6 patients (31%) achieved complete response (CR). One patient performed a subsequent allogeneic transplant. The median number of cycles to best response was 3 (extremes 1-8). There were no differences in ORR according to disease or patient characteristics. Four (30%) of the 13 transfusion-dependent patients achieved transfusion independence.
With a median follow-up of 10.3 months (m) (3.4-19.8), median overall survival (OS) was 8.7m, with an OS at 1 year (1y-OS) of 47%±24%. Excluding the 3 patients treated in 1L there was no difference in OS depending on disease status/previous treatment. 1y-OS was significantly better in patients who achieved ORR (83%±30% vs 12%±22%; p<0.001). The median event-free survival (EFS) was 6.9 m (95%CI 2.01-11.78), being significantly better in the group that achieved ORR compared to the rest (1y-OS :74%±32% vs 10%±18%; p<0.001) and better in those who achieved CR (1y-OS: 100% vs 11%±20%; p=0.001). Remarkable the three patients treated after alloSCT are alive and disease free at time of reporting.
There were no treatment related deaths. Regarding specific toxicities of ENA 4(21%) cases presented leukocytosis, 3 (15%) differentiation syndrome, 6 (31%) hyperbilirubinemia. There were 3 admissions due to toxicity and in 2 patients ENA was definitively discontinued due to toxicity.
Conclusion
The results of this study confirm both the effectiveness and the good drug tolerability reported with ENA in the Phase II study
Funding: PI16/01027 and PI19/01476 (JE; MDB)
Keyword(s): AML, Enasidenib