
Contributions
Abstract: EP472
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Combination of venetoclax (Ven), a specific BCL-2 inhibitor, with hypomethylating agents (HMA) provides a clinical benefit to newly-diagnosed acute myeloid leukaemia (AML) patients ineligible for standard chemotherapy, with a reported overall response rate over 60% and a low early death rate, improving previous results obtained with HMA monotherapy (DiNardo CD, et al., NEJM 2020). Moreover, role of VenHMA is being explored in other clinical settings. In 2019 we introduced in our institution this regimen for both patients with newly-diagnosed AML (ND-AML) ineligible patients for standard chemotherapy and R/R AML without alternative salvage options.
Aims
Herein, we present the outcome of the patients treated with VenHMA, focusing on the analysis of possible predictors of clinically meaningful benefit.
Methods
We analyzed the overall response and follow-up of 51 patients diagnosed with AML and mixed-phenotype acute leukaemia (MPAL, n=2) who received a combination of off-label venetoclax (400mg/24h; initial ramp-up 100-200-400mg in 3 consecutive days, with dose adjustment in patients who also received CYP3A4 inhibitors), with standard-dose azacytidine (75mg/m2) or decitabine (20mg/m2) from March 2019. Response to treatment was evaluated following the European LeukaemiaNet 2017 criteria. Effect of response in overall survival was evaluated as a time-dependent variable (Mantel-Byar method, Simon-Makuch survival plot). Event was defined as death, progressive disease or refractoriness to treatment.
Results
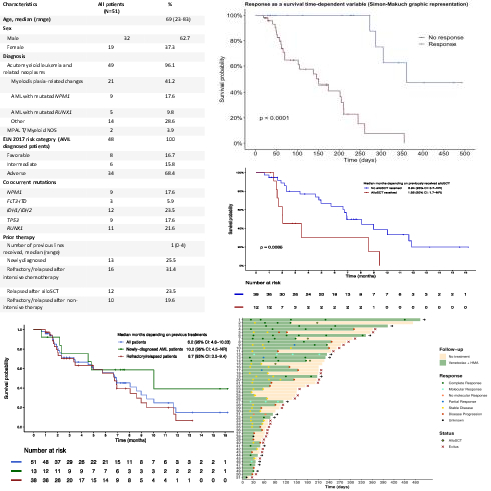
Characteristics of our cohort are described in table 1. Notably, our cohort was enriched with high-risk ELN subtypes, in two thirds of patients. Median number of VenHMA cycles received was two (range 1-11), with on-going treatment in 14 patients. Early mortality in the first 30 days was only 2% (1 patient). Response rate (Complete response (CR) + complete response without haematological recovery (CRi) + partial response (PR)) was 54% in ND-AML (CR= 23%) and 29% in R/R AML (CR= 12%) with a median of one cycle to achieve the best response (range: 1-5). Four patients were treated in a molecular-MRD positive status, 3 of them achieving a molecular response (3/3 NPM1mut AML). Four patients proceeded to an allogeneic haematopoietic cell transplantation (alloHSCT). Median overall survival in the cohort was 6.9 months ( 1.52). Median overall survival was respectively 10 months ( 2.78) in ND-AML patients and 6.7 months ( 1.8) in R/R AML patients. 6-month overall survival was 56.2% ( 14.9) and event-free survival was 6.6 months. Response to treatment after two cycles, estimated with Mantel-Byar method, segregated two groups of patients, with an overall survival of 12 months in patients who achieved a CR/CRi versus 5 months in non-responders (p<0.0001). Furthermore, patients treated after alloHSCT relapse presented a dismal outcome (6.94 vs. 1.88 months, p<0.001). There were no other significant clinical or biological variables able to predict outcome.
Conclusion
VenHMA is a highly active low-intensity regimen associated to a relatively low toxicity profile, providing a meaningful antileukaemic effect in a subset of patients not considered appropriate for higher intensity regimens, including the possibility to bridge some patients to alloHSCT. Despite the precise subset of patients who can benefit from this combination is still uncertain, especially in the R/R AML setting, its role in post-alloHSCT failure seems very limited. Early scope of response allows a rapid identification of those patients who benefit from continued treatment with VenHMA.
Keyword(s): AML, Immune therapy
Abstract: EP472
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Combination of venetoclax (Ven), a specific BCL-2 inhibitor, with hypomethylating agents (HMA) provides a clinical benefit to newly-diagnosed acute myeloid leukaemia (AML) patients ineligible for standard chemotherapy, with a reported overall response rate over 60% and a low early death rate, improving previous results obtained with HMA monotherapy (DiNardo CD, et al., NEJM 2020). Moreover, role of VenHMA is being explored in other clinical settings. In 2019 we introduced in our institution this regimen for both patients with newly-diagnosed AML (ND-AML) ineligible patients for standard chemotherapy and R/R AML without alternative salvage options.
Aims
Herein, we present the outcome of the patients treated with VenHMA, focusing on the analysis of possible predictors of clinically meaningful benefit.
Methods
We analyzed the overall response and follow-up of 51 patients diagnosed with AML and mixed-phenotype acute leukaemia (MPAL, n=2) who received a combination of off-label venetoclax (400mg/24h; initial ramp-up 100-200-400mg in 3 consecutive days, with dose adjustment in patients who also received CYP3A4 inhibitors), with standard-dose azacytidine (75mg/m2) or decitabine (20mg/m2) from March 2019. Response to treatment was evaluated following the European LeukaemiaNet 2017 criteria. Effect of response in overall survival was evaluated as a time-dependent variable (Mantel-Byar method, Simon-Makuch survival plot). Event was defined as death, progressive disease or refractoriness to treatment.
Results
Characteristics of our cohort are described in table 1. Notably, our cohort was enriched with high-risk ELN subtypes, in two thirds of patients. Median number of VenHMA cycles received was two (range 1-11), with on-going treatment in 14 patients. Early mortality in the first 30 days was only 2% (1 patient). Response rate (Complete response (CR) + complete response without haematological recovery (CRi) + partial response (PR)) was 54% in ND-AML (CR= 23%) and 29% in R/R AML (CR= 12%) with a median of one cycle to achieve the best response (range: 1-5). Four patients were treated in a molecular-MRD positive status, 3 of them achieving a molecular response (3/3 NPM1mut AML). Four patients proceeded to an allogeneic haematopoietic cell transplantation (alloHSCT). Median overall survival in the cohort was 6.9 months ( 1.52). Median overall survival was respectively 10 months ( 2.78) in ND-AML patients and 6.7 months ( 1.8) in R/R AML patients. 6-month overall survival was 56.2% ( 14.9) and event-free survival was 6.6 months. Response to treatment after two cycles, estimated with Mantel-Byar method, segregated two groups of patients, with an overall survival of 12 months in patients who achieved a CR/CRi versus 5 months in non-responders (p<0.0001). Furthermore, patients treated after alloHSCT relapse presented a dismal outcome (6.94 vs. 1.88 months, p<0.001). There were no other significant clinical or biological variables able to predict outcome.

Conclusion
VenHMA is a highly active low-intensity regimen associated to a relatively low toxicity profile, providing a meaningful antileukaemic effect in a subset of patients not considered appropriate for higher intensity regimens, including the possibility to bridge some patients to alloHSCT. Despite the precise subset of patients who can benefit from this combination is still uncertain, especially in the R/R AML setting, its role in post-alloHSCT failure seems very limited. Early scope of response allows a rapid identification of those patients who benefit from continued treatment with VenHMA.
Keyword(s): AML, Immune therapy


