Contributions
Abstract: EP471
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
ENA is approved in the US for adult pts with relapsed/refractory (R/R) mIDH2 AML. Azacitidine (AZA) is a hypomethylating agent that prolongs survival vs. conventional care regimens in older unfit pts with newly diagnosed (ND) AML. In vitro, combination ENA + AZA enhances cell differentiation. Venetoclax (VEN), a small molecule BCL2 inhibitor (i) is also effective in mIDH2 AML related to 2-hydroxyglutarate mediated inhibition of cytochrome C oxidase. In preclinical models, the combination of ENA+VEN demonstrates synergistic anti-leukemic activity.
Aims
Evaluate the efficacy and safety of combination ENA + AZA +/- VEN in ND and R/R mIDH2 AML
Methods
Patients (pts) with documented mIDH2 AML: ≥ 60 yrs with ND AML and ineligible for intensive chemotherapy; >18 yrs with secondary AML (sAML) and/or R/R AML were enrolled. All pts received AZA 75 mg/m2/d x 7 d/cycle and ENA 100 mg QD continuously on NCT03683433. Cytoreduction prior to start, and concomitant BCL2 i and FLT3 i were allowed as indicated. Primary objective was overall response rate (ORR) and secondary objectives included safety and overall survival (OS).
Results
This high-risk cohort included 26 patients with a median age of 68 years (46% of pts ≥70 yrs), 28% pts had ECOG PS ≥2, 42% had received ≥2 salvage therapy, and 41% pts had ELN2017 adverse-risk AML (Table 1). 30 and 60-day mortality were both 0 for ND pts,5% and 5%, respectively, in R/R pts. Treatment-emergent adverse events (TEAE) of grade 3-4 were reported in 22/26 pts (85%), with the most frequent TEAEs including febrile neutropenia (23%), indirect hyperbilirubinemia (35%), and vomiting and diarrhea (12% each). Most AEs were manageable without dose interruption. Differentiation syndrome was reported in 2 pts (8%), occurring on days 18 and 31 of treatment.
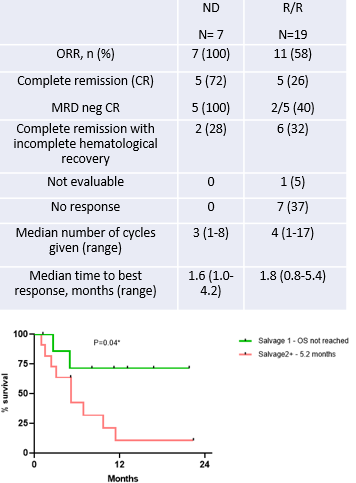
The CR/CRi rate in ND AML was 100% (n=7/7), and in R/R AML was 58% (n=11/19) (Table). CR/CRi rate was 75% in pts in first relapse (n=6/8); and 45% in ≥2 relapse (n=5/11). CR/CRi rate in pts with prior HMA was 67% (n=6/9), in pts with prior ENA was 75% (n=3/4), and in pts with prior stem cell transplantation (SCT) was 60% (n=3/5). 39% of all responders (n=7/18) achieved MRD negativity by flow cytometry (MRD neg FCM) and MRD neg status was associated with longer OS (NR vs 6.9 mo, HR 0.16, 95% CI 0.05-0.5, p=0.04). At median follow-up of 13.1 months (mo), the 1-year OS in ND AML was 85%, in first relapse was 75%, in ≥2 relapse was 10%. Median OS was not reached in ND AML; Pts treated in first relapse had a significantly superior OS than those in ≥2 relapse. (median OS not reached vs 5.2 mo; HR 0.24, 95% CI 0.07-0.79, p=0.04, Fig.)
In the mIDH2 R/R AML cohort, 7 pts received the ENA+AZA+VEN triplet, among which 4 had received prior HMA and one had received prior HMA and ENA separately. None had received prior VEN. The CR/CRi rate was 86% (n=6/7) with 33% MRDneg FCM (n=2/6) with one patient proceeding to SCT. At a median follow up of 11.2 mo, median OS was not reached, and 6-mo OS was 70% in those who received ENA+AZA+VEN.
With a median follow-up of 13.1 mo, 38% (n=9) remain on treatment and 54% alive (n=14) at last follow up. Reasons for treatment discontinuation(n=17): relapse (n=1, 4%), SCT (n=4, 15%), death (n=8, 31%), refractory disease (n=3, 12%), and personal choice (n=1,4%).
Conclusion
AZA+ENA is an effective therapy for IDH2-mutated AML.ENA+AZA+VEN may be an effective combination therapy for mIDH2 R/R AML, even in patients having received prior enasidenib.
Keyword(s): AG-221, AML, Azacitidine, Relapsed acute myeloid leukemia
Abstract: EP471
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
ENA is approved in the US for adult pts with relapsed/refractory (R/R) mIDH2 AML. Azacitidine (AZA) is a hypomethylating agent that prolongs survival vs. conventional care regimens in older unfit pts with newly diagnosed (ND) AML. In vitro, combination ENA + AZA enhances cell differentiation. Venetoclax (VEN), a small molecule BCL2 inhibitor (i) is also effective in mIDH2 AML related to 2-hydroxyglutarate mediated inhibition of cytochrome C oxidase. In preclinical models, the combination of ENA+VEN demonstrates synergistic anti-leukemic activity.
Aims
Evaluate the efficacy and safety of combination ENA + AZA +/- VEN in ND and R/R mIDH2 AML
Methods
Patients (pts) with documented mIDH2 AML: ≥ 60 yrs with ND AML and ineligible for intensive chemotherapy; >18 yrs with secondary AML (sAML) and/or R/R AML were enrolled. All pts received AZA 75 mg/m2/d x 7 d/cycle and ENA 100 mg QD continuously on NCT03683433. Cytoreduction prior to start, and concomitant BCL2 i and FLT3 i were allowed as indicated. Primary objective was overall response rate (ORR) and secondary objectives included safety and overall survival (OS).
Results
This high-risk cohort included 26 patients with a median age of 68 years (46% of pts ≥70 yrs), 28% pts had ECOG PS ≥2, 42% had received ≥2 salvage therapy, and 41% pts had ELN2017 adverse-risk AML (Table 1). 30 and 60-day mortality were both 0 for ND pts,5% and 5%, respectively, in R/R pts. Treatment-emergent adverse events (TEAE) of grade 3-4 were reported in 22/26 pts (85%), with the most frequent TEAEs including febrile neutropenia (23%), indirect hyperbilirubinemia (35%), and vomiting and diarrhea (12% each). Most AEs were manageable without dose interruption. Differentiation syndrome was reported in 2 pts (8%), occurring on days 18 and 31 of treatment.
The CR/CRi rate in ND AML was 100% (n=7/7), and in R/R AML was 58% (n=11/19) (Table). CR/CRi rate was 75% in pts in first relapse (n=6/8); and 45% in ≥2 relapse (n=5/11). CR/CRi rate in pts with prior HMA was 67% (n=6/9), in pts with prior ENA was 75% (n=3/4), and in pts with prior stem cell transplantation (SCT) was 60% (n=3/5). 39% of all responders (n=7/18) achieved MRD negativity by flow cytometry (MRD neg FCM) and MRD neg status was associated with longer OS (NR vs 6.9 mo, HR 0.16, 95% CI 0.05-0.5, p=0.04). At median follow-up of 13.1 months (mo), the 1-year OS in ND AML was 85%, in first relapse was 75%, in ≥2 relapse was 10%. Median OS was not reached in ND AML; Pts treated in first relapse had a significantly superior OS than those in ≥2 relapse. (median OS not reached vs 5.2 mo; HR 0.24, 95% CI 0.07-0.79, p=0.04, Fig.)
In the mIDH2 R/R AML cohort, 7 pts received the ENA+AZA+VEN triplet, among which 4 had received prior HMA and one had received prior HMA and ENA separately. None had received prior VEN. The CR/CRi rate was 86% (n=6/7) with 33% MRDneg FCM (n=2/6) with one patient proceeding to SCT. At a median follow up of 11.2 mo, median OS was not reached, and 6-mo OS was 70% in those who received ENA+AZA+VEN.
With a median follow-up of 13.1 mo, 38% (n=9) remain on treatment and 54% alive (n=14) at last follow up. Reasons for treatment discontinuation(n=17): relapse (n=1, 4%), SCT (n=4, 15%), death (n=8, 31%), refractory disease (n=3, 12%), and personal choice (n=1,4%).
Conclusion
AZA+ENA is an effective therapy for IDH2-mutated AML.ENA+AZA+VEN may be an effective combination therapy for mIDH2 R/R AML, even in patients having received prior enasidenib.
Keyword(s): AG-221, AML, Azacitidine, Relapsed acute myeloid leukemia