
Contributions
Abstract: EP470
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Multiple studies have revealed the prognostic utility of combining FLT3-ITD and NPM1 mutation status to identify patients benefiting from allogeneic stem cell transplantation (alloSCT) in first remission (CR1). In recent years, analysis of peripheral blood measurable residual disease (MRD) has superseded genetic risk stratification for NPM1 mutated (NPM1+) AML within the UK. This strategy aims to reduce SCT-related toxicity, but requires demonstration of chemo-sensitivity at relapse for higher-risk genotypes, in particular patients with FLT3-ITD mutations.
Aims
We aimed to assess clinical outcomes in NPM1+ AML, comparing the impact of FLT3-ITD mutations in patients receiving consolidation with chemotherapy versus alloSCT in CR1 and reviewing responses to second-line therapies at relapse.
Methods
Retrospective analysis identified 115 patients with NPM1+ AML eligible for intensive therapy between 2014-2020. The median age was 59y, 53% were male and median follow-up was 3.2y. FLT3-ITD mutations were detected in 38% of cases (NPM1+/FLT3-ITD+, n=44), for the purposes of this study, FLT3 tyrosine kinase domain mutations (n=16) were classified as FLT3-ITD negative (FLT3-ITD-ve) AML.
Results
CR rates were comparable for both FLT3-ITD+ and FLT3-ITD-ve cases following induction therapy (91% vs 94%, p=0.48). Within FLT3-ITD+ cases, 32% (n=14) underwent alloSCT in CR1, compared with only 11% (n=8) of FLT3-ITD-ve cases. Notably, FLT3-ITD+ AML demonstrated lower survival (hazard ratio, 2.18, p=0.02) and a higher risk of relapse (HR 1.88, p=0.03) compared to FLT3-ITD-ve cases, regardless of therapy.
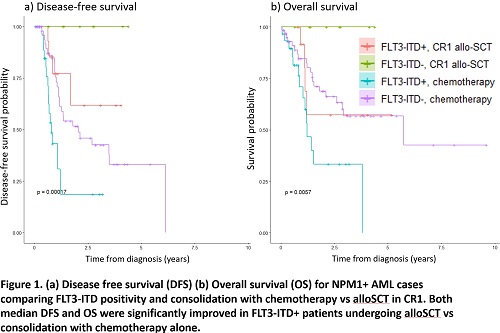
Further analysis revealed a significantly higher risk of relapse (HR 3.84, p=0.02), inferior median disease-free survival (0.80y vs not reached, p=0.002) and median overall survival (1.21y vs not reached, p=0.02) for FLT3-ITD+ cases receiving chemotherapy consolidation, compared with alloSCT in CR1. Interestingly, the small number of FLT3-ITD-ve cases undergoing alloSCT in CR1 (n=8) also demonstrated superior outcomes, with no deaths or relapses, compared with those receiving chemotherapy alone (median DFS not reached vs 2.0y, p=0.03 and median OS not reached vs 5.7y, p=0.34), Fig 1 a) and b).
Relapse was observed in 47% of patients receiving chemotherapy alone (n=44); of these cases 39% (n=17) were FLT3-ITD+ and exhibited a significantly shorter median time to relapse vs FLT3-ITD-ve cases (247 days vs 413 days, p=0.01). In contrast, only 18% (n=4) of all patients undergoing alloSCT in CR1 relapsed and each harboured FLT3-ITD mutations. Importantly, 52% (n=14) of FLT3-ITD-ve cases achieved a second remission (CR2) with chemotherapy, while only 24% (n=5) of FLT3-ITD+ cases were able to reach CR2 (p=0.049 [data precede availability of Gilteritinib at relapse]). These findings highlight the chemo-refractory nature of relapsed FLT3-ITD mutated AML, with a significantly reduced median survival post-relapse, compared with FLT3-ITD-ve cases (0.5y vs 1.1y, p=0.03).
Conclusion
This study demonstrates favourable survival for patients with NPM1+/FLT3-ITD+ AML undergoing alloSCT in CR1. Significantly higher relapse rates were observed in FLT3-ITD+ cases treated with chemotherapy alone, followed by poor responses to salvage chemotherapy and fewer patients achieving CR2. Collectively, our data suggest that alloSCT consolidation should be prioritised in NPM1+/FLT3-ITD+ AML to achieve improved long-term outcomes within this higher risk cohort.
Keyword(s): Acute myeloid leukemia, Allogeneic hematopoietic stem cell transplant, Flt3-ITD
Abstract: EP470
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Multiple studies have revealed the prognostic utility of combining FLT3-ITD and NPM1 mutation status to identify patients benefiting from allogeneic stem cell transplantation (alloSCT) in first remission (CR1). In recent years, analysis of peripheral blood measurable residual disease (MRD) has superseded genetic risk stratification for NPM1 mutated (NPM1+) AML within the UK. This strategy aims to reduce SCT-related toxicity, but requires demonstration of chemo-sensitivity at relapse for higher-risk genotypes, in particular patients with FLT3-ITD mutations.
Aims
We aimed to assess clinical outcomes in NPM1+ AML, comparing the impact of FLT3-ITD mutations in patients receiving consolidation with chemotherapy versus alloSCT in CR1 and reviewing responses to second-line therapies at relapse.
Methods
Retrospective analysis identified 115 patients with NPM1+ AML eligible for intensive therapy between 2014-2020. The median age was 59y, 53% were male and median follow-up was 3.2y. FLT3-ITD mutations were detected in 38% of cases (NPM1+/FLT3-ITD+, n=44), for the purposes of this study, FLT3 tyrosine kinase domain mutations (n=16) were classified as FLT3-ITD negative (FLT3-ITD-ve) AML.
Results
CR rates were comparable for both FLT3-ITD+ and FLT3-ITD-ve cases following induction therapy (91% vs 94%, p=0.48). Within FLT3-ITD+ cases, 32% (n=14) underwent alloSCT in CR1, compared with only 11% (n=8) of FLT3-ITD-ve cases. Notably, FLT3-ITD+ AML demonstrated lower survival (hazard ratio, 2.18, p=0.02) and a higher risk of relapse (HR 1.88, p=0.03) compared to FLT3-ITD-ve cases, regardless of therapy.
Further analysis revealed a significantly higher risk of relapse (HR 3.84, p=0.02), inferior median disease-free survival (0.80y vs not reached, p=0.002) and median overall survival (1.21y vs not reached, p=0.02) for FLT3-ITD+ cases receiving chemotherapy consolidation, compared with alloSCT in CR1. Interestingly, the small number of FLT3-ITD-ve cases undergoing alloSCT in CR1 (n=8) also demonstrated superior outcomes, with no deaths or relapses, compared with those receiving chemotherapy alone (median DFS not reached vs 2.0y, p=0.03 and median OS not reached vs 5.7y, p=0.34), Fig 1 a) and b).
Relapse was observed in 47% of patients receiving chemotherapy alone (n=44); of these cases 39% (n=17) were FLT3-ITD+ and exhibited a significantly shorter median time to relapse vs FLT3-ITD-ve cases (247 days vs 413 days, p=0.01). In contrast, only 18% (n=4) of all patients undergoing alloSCT in CR1 relapsed and each harboured FLT3-ITD mutations. Importantly, 52% (n=14) of FLT3-ITD-ve cases achieved a second remission (CR2) with chemotherapy, while only 24% (n=5) of FLT3-ITD+ cases were able to reach CR2 (p=0.049 [data precede availability of Gilteritinib at relapse]). These findings highlight the chemo-refractory nature of relapsed FLT3-ITD mutated AML, with a significantly reduced median survival post-relapse, compared with FLT3-ITD-ve cases (0.5y vs 1.1y, p=0.03).

Conclusion
This study demonstrates favourable survival for patients with NPM1+/FLT3-ITD+ AML undergoing alloSCT in CR1. Significantly higher relapse rates were observed in FLT3-ITD+ cases treated with chemotherapy alone, followed by poor responses to salvage chemotherapy and fewer patients achieving CR2. Collectively, our data suggest that alloSCT consolidation should be prioritised in NPM1+/FLT3-ITD+ AML to achieve improved long-term outcomes within this higher risk cohort.
Keyword(s): Acute myeloid leukemia, Allogeneic hematopoietic stem cell transplant, Flt3-ITD


