
Contributions
Abstract: EP468
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
The use of FLT3 inhibitors (FLT3i) has improved overall survival (OS) in patients with newly diagnosed FLT3-mutated acute myeloid leukemia (AML). However, the role of FLT3i in patients with very low FLT3-ITD allelic burden is unclear.
Aims
To analyze the role and impact of FLT3i’s, NPM1 co-mutation, and allogeneic stem cell transplantation (ASCT) in patients with a very low FLT3 allelic burden.
Methods
A multiplex PCR-based DNA analysis followed by capillary electrophoresis was utilized to detect FLT3-ITD mutations at diagnosis and relapse. Allelic frequency (AF) is defined as the ratio of mutated FLT3-ITD divided by wild type plus mutated FLT3-ITD. We identified 50 patients with newly diagnosed AML (excluding core-binding factor AML and acute promyelocytic leukemia) from 2012 – 2020 at our institusion who had a FLT3-ITD AF ≤0.10 (i.e. ≤10%) at baseline.
Results
Of the 50 patients, 30 (60%) received frontline therapy not including a FLT3i (no FLT3i cohort), and 20 with a FLT3i (FLT3i cohort) containing regimen. Patients in the FLT3i cohort were younger than the no FLT3i cohort (median age 58 [28-83] vs. 67 [23-84] years old, p=0.03), and more often received intensive (cytarabine and antracycline based) induction (80% vs 43%, p=0.01). Otherwise, there were no other significant baseline clinical difference between the two cohorts. Baseline median ITD AFs were not significantly different between FLT3i (0.05) and no FLT3i (0.03) cohorts. Of the 20 patients in FLT3i group, 19 (95%) received sorafenib, and 1 (5%) received quizartinib based therapies.
Overall, 37 of 50 (74%) patients achieved a complete remission (CR). With a median follow-up of 42 months, 12 (32%) patients relapsed. The incidence of FLT3-ITD positivity at relapse was 42% (5 of 12). All FLT3 positive relapses were identified in the no FLT3i cohort (5 of 8), with no FLT3 positive relapses observed in the FLT3i cohort (0 of 4): 63% vs. 0%, respectively (p=0.04). There was a trend for better OS in the FLT3i cohort compared with no FLT3i cohort, not reached vs. 17 months (p=0.31). The 5-year OS rate was improved in patients who received a FLT3i with induction and ASCT compared with patients who did not receive a FLT3i with induction and did not receive ASCT (100% vs 27%, p=0.08).
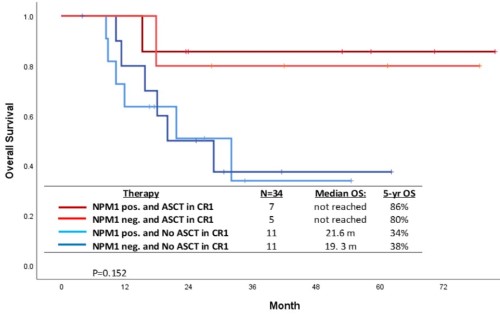
Overall, 14 patients underwent ASCT (6 FLT3i and 8 no FLT3i cohort) in first remission with a median time to ASCT from induction of 114 days (range 79-230 days). A landmark analysis (at 3-month post ASCT) showed that 5-year OS rate was superior in patients who underwent ASCT in CR1 (N=14) compared with those who did not (N=22), 85% vs. 34%, respectively (p=0.01). Interestingly in this low FLT3-ITD VAF (≤0.1) population the 5-year OS in patients with NPM1 co-mutation who received ASCT (N=7), NPM1 without ASCT (N=11), no NPM1 with ASCT (N=5), and no NPM1 without ASCT (N=11) were 86%, 34%, 80%, and 38%, respectively (p=0.15) [Figure].
Conclusion
The use of FLT3i reduced the incidence of FLT3 positive relapses. The use of FLT3i with induction and ASCT showed improved OS in AML patients with very low FLT3-ITD AFs. Even among patients with very low FLT3-ITD VAF and NPM1 comutations, ASCT appeared to improve 5-year OS (86% vs 34%). We recommend that all FLT3-ITDm patients receive a FLT3i with induction and be considered for ASCT in CR1 irrespective of baseline FLT3 AF and/or NPM1 co-mutation status. To better understand the impact of very low FLT3 burden, future clinical trials investigating FLT3i should include low VAF FLT3-mutated AML patients.
Keyword(s): AML, FLT3
Abstract: EP468
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
The use of FLT3 inhibitors (FLT3i) has improved overall survival (OS) in patients with newly diagnosed FLT3-mutated acute myeloid leukemia (AML). However, the role of FLT3i in patients with very low FLT3-ITD allelic burden is unclear.
Aims
To analyze the role and impact of FLT3i’s, NPM1 co-mutation, and allogeneic stem cell transplantation (ASCT) in patients with a very low FLT3 allelic burden.
Methods
A multiplex PCR-based DNA analysis followed by capillary electrophoresis was utilized to detect FLT3-ITD mutations at diagnosis and relapse. Allelic frequency (AF) is defined as the ratio of mutated FLT3-ITD divided by wild type plus mutated FLT3-ITD. We identified 50 patients with newly diagnosed AML (excluding core-binding factor AML and acute promyelocytic leukemia) from 2012 – 2020 at our institusion who had a FLT3-ITD AF ≤0.10 (i.e. ≤10%) at baseline.
Results
Of the 50 patients, 30 (60%) received frontline therapy not including a FLT3i (no FLT3i cohort), and 20 with a FLT3i (FLT3i cohort) containing regimen. Patients in the FLT3i cohort were younger than the no FLT3i cohort (median age 58 [28-83] vs. 67 [23-84] years old, p=0.03), and more often received intensive (cytarabine and antracycline based) induction (80% vs 43%, p=0.01). Otherwise, there were no other significant baseline clinical difference between the two cohorts. Baseline median ITD AFs were not significantly different between FLT3i (0.05) and no FLT3i (0.03) cohorts. Of the 20 patients in FLT3i group, 19 (95%) received sorafenib, and 1 (5%) received quizartinib based therapies.
Overall, 37 of 50 (74%) patients achieved a complete remission (CR). With a median follow-up of 42 months, 12 (32%) patients relapsed. The incidence of FLT3-ITD positivity at relapse was 42% (5 of 12). All FLT3 positive relapses were identified in the no FLT3i cohort (5 of 8), with no FLT3 positive relapses observed in the FLT3i cohort (0 of 4): 63% vs. 0%, respectively (p=0.04). There was a trend for better OS in the FLT3i cohort compared with no FLT3i cohort, not reached vs. 17 months (p=0.31). The 5-year OS rate was improved in patients who received a FLT3i with induction and ASCT compared with patients who did not receive a FLT3i with induction and did not receive ASCT (100% vs 27%, p=0.08).
Overall, 14 patients underwent ASCT (6 FLT3i and 8 no FLT3i cohort) in first remission with a median time to ASCT from induction of 114 days (range 79-230 days). A landmark analysis (at 3-month post ASCT) showed that 5-year OS rate was superior in patients who underwent ASCT in CR1 (N=14) compared with those who did not (N=22), 85% vs. 34%, respectively (p=0.01). Interestingly in this low FLT3-ITD VAF (≤0.1) population the 5-year OS in patients with NPM1 co-mutation who received ASCT (N=7), NPM1 without ASCT (N=11), no NPM1 with ASCT (N=5), and no NPM1 without ASCT (N=11) were 86%, 34%, 80%, and 38%, respectively (p=0.15) [Figure].

Conclusion
The use of FLT3i reduced the incidence of FLT3 positive relapses. The use of FLT3i with induction and ASCT showed improved OS in AML patients with very low FLT3-ITD AFs. Even among patients with very low FLT3-ITD VAF and NPM1 comutations, ASCT appeared to improve 5-year OS (86% vs 34%). We recommend that all FLT3-ITDm patients receive a FLT3i with induction and be considered for ASCT in CR1 irrespective of baseline FLT3 AF and/or NPM1 co-mutation status. To better understand the impact of very low FLT3 burden, future clinical trials investigating FLT3i should include low VAF FLT3-mutated AML patients.
Keyword(s): AML, FLT3


