
Contributions
Abstract: EP467
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
DNA-hypomethylating agents are the backbone for non-intensive combination treatments of AML/MDS patients (pts). In elderly AML pts, the decitabine (DAC) + all-trans retinoic acid (ATRA) combination resulted in an improved response rate and survival compared to DAC without ATRA (DECIDER, Lübbert et al., J. Clin. Oncol. 2020), also in pts with prior hematologic disorder (mostly MDS).
Aims
We hypothesized that the outcome of pts with oligoblastic AML may also be improved by the addition of ATRA to DAC. Therefore, pts from the DECIDER cohort with 20-30% bone marrow blasts were analyzed for clinical outcome in this exploratory subgroup analysis.
Methods
Key inclusion criteria: newly diagnosed pts >60 years (yr), unfit for induction with non-M3 AML, ECOG performance status (PS) 0-2. Treatment: DAC 20 mg/m2 day 1-5 (treatment arms A/B/C/D), ATRA p.o. day 6-28 (arms C/D), VPA p.o. continuously from day 6 (arms B/D) of each 28-day course (repeated until relapse/progression, prohibitive toxicity, withdrawal or death). Key endpoints: objective response rate (ORR): CR/CRi/PR, overall (OS) and event-free survival (EFS). For a power of 80% (test in this phase II study at 1-sided alpha=0.1) for an increase of ORR to 40% with ATRA or VPA, 176 pts were necessary, planned sample size 200. Between 12/2011 and 2/2015, 200 pts were randomized and treated. Efficacy analyses were performed in the intention-to-treat (ITT) population. ATRA was investigated by comparing arms C+D vs arms A+B, VPA by comparing arms B+D vs arms A+C, ORR was analyzed with logistic regression estimating odds ratios (OR), OS/EFS with Cox regression estimating hazard ratios (HR), each with 95% confidence intervals (CI), and presented with descriptive two-sided p values of the tests of no treatment effect.
Results
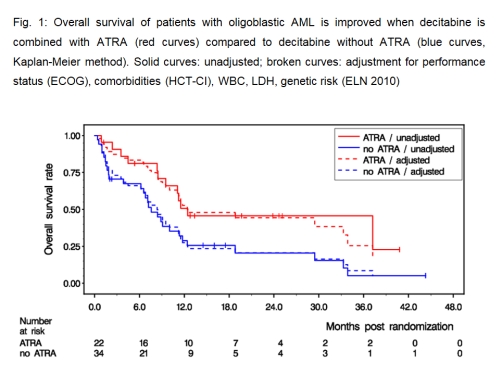
Bone marrow blasts were 20-30% (median, 25%) defining oligoblastic AML in 56/200 pts of the DECIDER cohort. The number of pts in the randomized arms were: 13 in arm A, 21 in arm B, 9 in arm C, 13 in arm D. Baseline pt characteristics: male 77%, median age: 75 yr (range 61-88), median WBC: 3400/µl (range 500-52,600), adverse genetics (ELN 2010) present in 25%, ECOG 2 in 13%, comorbidities (HCT-CI) ≥ 3 in 48%, AHD in 68%, tAML in 11%. A median of 5 DAC courses were administered (per arm: 2/5/11/4). Six pts attained a CR, 7 pts a CRi, and 1 pt a PR, resulting in an ORR of 25% (arm A: 7.7%, arm B: 28.6%, arm C: 33.3%, arm D: 30.8%, respectively). Effect on ORR of ATRA vs no ATRA (31.8 vs 20.6%): OR 1.85, CI [0.54,6.37], p=0.33; and of VPA vs no VPA (29.4 vs 18.2%): OR 1.93, CI [0.51,7.24], p=0.33. With 40 deaths out of 56 pts, median OS was 9.5 mths (arm A: 7.6 mths, arm B: 8.9 mths, arm C: 37.2 mths, arm D: 11.2 mths, respectively). Effect on OS of ATRA vs. no ATRA (12.5 vs 7.6 mths median OS, Fig. 1): HR 0.47, CI [0.24,0.94], p=0.032 (after adjustment for PS, HCT-CI, WBC, LDH, genetic risk: HR 0.42, CI [0.19,0.90], p=0.025); and of VPA vs. no VPA (10.0 vs 8.4 mths median OS): HR 0.99, CI [0.51,1.92], p=0.98. A comparable benefit on EFS of ATRA vs. no ATRA (but not VPA vs. no VPA) was observed.
Conclusion
In elderly pts with oligoblastic AML ineligible for induction chemotherapy, the addition of ATRA, but not VPA, to DAC resulted in a clinically meaningful survival benefit. It is tempting to speculate that the combination of an HMA with a retinoid may also be active in MDS pts with excess of blasts. In this regard, a novel and potent RARα receptor agonist (SY-1425) is under clinical development combined with an HMA in AML/MDS (De Botton S. et al., ASH Abs. 112, 2020).
Keyword(s): Acute myeloid leukemia, Decitabine, Epigenetic, Retinoic acid
Abstract: EP467
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
DNA-hypomethylating agents are the backbone for non-intensive combination treatments of AML/MDS patients (pts). In elderly AML pts, the decitabine (DAC) + all-trans retinoic acid (ATRA) combination resulted in an improved response rate and survival compared to DAC without ATRA (DECIDER, Lübbert et al., J. Clin. Oncol. 2020), also in pts with prior hematologic disorder (mostly MDS).
Aims
We hypothesized that the outcome of pts with oligoblastic AML may also be improved by the addition of ATRA to DAC. Therefore, pts from the DECIDER cohort with 20-30% bone marrow blasts were analyzed for clinical outcome in this exploratory subgroup analysis.
Methods
Key inclusion criteria: newly diagnosed pts >60 years (yr), unfit for induction with non-M3 AML, ECOG performance status (PS) 0-2. Treatment: DAC 20 mg/m2 day 1-5 (treatment arms A/B/C/D), ATRA p.o. day 6-28 (arms C/D), VPA p.o. continuously from day 6 (arms B/D) of each 28-day course (repeated until relapse/progression, prohibitive toxicity, withdrawal or death). Key endpoints: objective response rate (ORR): CR/CRi/PR, overall (OS) and event-free survival (EFS). For a power of 80% (test in this phase II study at 1-sided alpha=0.1) for an increase of ORR to 40% with ATRA or VPA, 176 pts were necessary, planned sample size 200. Between 12/2011 and 2/2015, 200 pts were randomized and treated. Efficacy analyses were performed in the intention-to-treat (ITT) population. ATRA was investigated by comparing arms C+D vs arms A+B, VPA by comparing arms B+D vs arms A+C, ORR was analyzed with logistic regression estimating odds ratios (OR), OS/EFS with Cox regression estimating hazard ratios (HR), each with 95% confidence intervals (CI), and presented with descriptive two-sided p values of the tests of no treatment effect.
Results
Bone marrow blasts were 20-30% (median, 25%) defining oligoblastic AML in 56/200 pts of the DECIDER cohort. The number of pts in the randomized arms were: 13 in arm A, 21 in arm B, 9 in arm C, 13 in arm D. Baseline pt characteristics: male 77%, median age: 75 yr (range 61-88), median WBC: 3400/µl (range 500-52,600), adverse genetics (ELN 2010) present in 25%, ECOG 2 in 13%, comorbidities (HCT-CI) ≥ 3 in 48%, AHD in 68%, tAML in 11%. A median of 5 DAC courses were administered (per arm: 2/5/11/4). Six pts attained a CR, 7 pts a CRi, and 1 pt a PR, resulting in an ORR of 25% (arm A: 7.7%, arm B: 28.6%, arm C: 33.3%, arm D: 30.8%, respectively). Effect on ORR of ATRA vs no ATRA (31.8 vs 20.6%): OR 1.85, CI [0.54,6.37], p=0.33; and of VPA vs no VPA (29.4 vs 18.2%): OR 1.93, CI [0.51,7.24], p=0.33. With 40 deaths out of 56 pts, median OS was 9.5 mths (arm A: 7.6 mths, arm B: 8.9 mths, arm C: 37.2 mths, arm D: 11.2 mths, respectively). Effect on OS of ATRA vs. no ATRA (12.5 vs 7.6 mths median OS, Fig. 1): HR 0.47, CI [0.24,0.94], p=0.032 (after adjustment for PS, HCT-CI, WBC, LDH, genetic risk: HR 0.42, CI [0.19,0.90], p=0.025); and of VPA vs. no VPA (10.0 vs 8.4 mths median OS): HR 0.99, CI [0.51,1.92], p=0.98. A comparable benefit on EFS of ATRA vs. no ATRA (but not VPA vs. no VPA) was observed.

Conclusion
In elderly pts with oligoblastic AML ineligible for induction chemotherapy, the addition of ATRA, but not VPA, to DAC resulted in a clinically meaningful survival benefit. It is tempting to speculate that the combination of an HMA with a retinoid may also be active in MDS pts with excess of blasts. In this regard, a novel and potent RARα receptor agonist (SY-1425) is under clinical development combined with an HMA in AML/MDS (De Botton S. et al., ASH Abs. 112, 2020).
Keyword(s): Acute myeloid leukemia, Decitabine, Epigenetic, Retinoic acid


