Contributions
Abstract: EP466
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Recent FDA approvals of novel therapies and hypomethylating agents / venetoclax has transformed therapy of newly diagnosed older patients with AML. While achievement of complete remission (CR) has been the gold standard for AML response, novel therapies with different response dynamics warrant re-evaluation of response criteria. CR with partial hematological recovery (CRh, bone marrow blasts < 5%, ANC > 0.5 x 109 /L and platelet count >50 x 109 /L) has been employed as a surrogate marker for efficacy and clinical benefit.
Aims
We evaluated the impact of CRh on long-term outcomes of older pts with newly diagnosed AML treated with 10-day decitabine and venetoclax (DEC10-VEN, NCT03404193).
Methods
Patients with newly diagnosed AML ages >60 years, or secondary AML were included. Patients received decitabine 20 mg/m² IV for 10 days with oral venetoclax 400 mg daily for induction and decitabine for 5 days with daily venetoclax for consolidation. Definitions of best response were as following: CR (Bone marrow blasts < 5% blasts, absence of circulating blasts and blasts with Auer rods, absence of extramedullary disease and ANC > 1 x 109 /L with platelet count >100 x 109 /L), CR with incomplete hematologic recovery, CRi (All CR criteria but with residual cytopenia such as ANC < 1 x 109 /L or platelet count <100 x 109 /L) and morphological leukemia free state, MLFS (CR criteria without hematologic recovery). Outcomes of interest included overall survival (OS) and event-free survival (EFS). Full study protocol and interim results have been published previously (DiNardo et al. Lancet Haematol. 2020;7:e724-36.)
Results
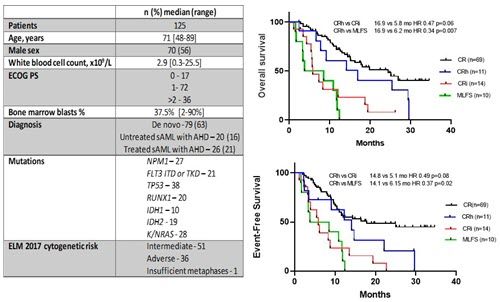
From January 2018 - February 2021 125 patients with a median age of 71 yrs (range 48-89) were included. 79 (63%) pts were newly diagnosed AML and 46 (37 %) had secondary AML with antecedent hematological disorder (Figure 1). 69 patients achieved CR (55%), 11 (8%) pts achieved CRh, 14 (11%) pts achieved CRi, 10 (8%) pts achieved MLFS and 21 (17%) pts were refractory. Of the 11 pts who met the criteria for CRh, 8 (73%) patients were previously classified as CRi and 3 (27%) as MLFS. Among 69 pts achieving CR, the median OS was 24.5 months (mo) and median EFS was 18.0 mo. CRh was achieved with a median of 1 (range 1-3) cycles. Median OS of CRh as compared to CRi was 16.9 vs 5.8 mo (HR 0.47, 95% CI 0.19-1.13, p=.06) and CRh vs MLFS was 16.9 vs 6.2 mo (HR 0.34, 95% CI 0.13-0.93, p=.007). Median EFS of pts with CRh vs CRi was 14.1 vs 5.8 mo (HR 0.49, 95%CI 0.21-1.14, p=.08) and CRh vs MLFS was 14.1 vs 6.2 mo (HR 0.37, 95% CI 0.14-1.01, p=.016). Pts achieving CRh defined a group with durable remission with duration of response of 6.0 vs 2.5 mo with CRi and 1 mo with MLFS. Additional analyses are ongoing and will be presented.
Conclusion
In pts with newly diagnosed AML and secondary AML treated with DEC10-VEN, pts achieving CRh had a trend towards improved OS and EFS compared to pts with CRi or MLFS. These findings support the use of CRh as an endpoint demonstrating clinical benefit for patients receiving venetoclax-based therapy. Larger analyses are warranted.
Keyword(s): Acute myeloid leukemia, Decitabine
Abstract: EP466
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Recent FDA approvals of novel therapies and hypomethylating agents / venetoclax has transformed therapy of newly diagnosed older patients with AML. While achievement of complete remission (CR) has been the gold standard for AML response, novel therapies with different response dynamics warrant re-evaluation of response criteria. CR with partial hematological recovery (CRh, bone marrow blasts < 5%, ANC > 0.5 x 109 /L and platelet count >50 x 109 /L) has been employed as a surrogate marker for efficacy and clinical benefit.
Aims
We evaluated the impact of CRh on long-term outcomes of older pts with newly diagnosed AML treated with 10-day decitabine and venetoclax (DEC10-VEN, NCT03404193).
Methods
Patients with newly diagnosed AML ages >60 years, or secondary AML were included. Patients received decitabine 20 mg/m² IV for 10 days with oral venetoclax 400 mg daily for induction and decitabine for 5 days with daily venetoclax for consolidation. Definitions of best response were as following: CR (Bone marrow blasts < 5% blasts, absence of circulating blasts and blasts with Auer rods, absence of extramedullary disease and ANC > 1 x 109 /L with platelet count >100 x 109 /L), CR with incomplete hematologic recovery, CRi (All CR criteria but with residual cytopenia such as ANC < 1 x 109 /L or platelet count <100 x 109 /L) and morphological leukemia free state, MLFS (CR criteria without hematologic recovery). Outcomes of interest included overall survival (OS) and event-free survival (EFS). Full study protocol and interim results have been published previously (DiNardo et al. Lancet Haematol. 2020;7:e724-36.)
Results
From January 2018 - February 2021 125 patients with a median age of 71 yrs (range 48-89) were included. 79 (63%) pts were newly diagnosed AML and 46 (37 %) had secondary AML with antecedent hematological disorder (Figure 1). 69 patients achieved CR (55%), 11 (8%) pts achieved CRh, 14 (11%) pts achieved CRi, 10 (8%) pts achieved MLFS and 21 (17%) pts were refractory. Of the 11 pts who met the criteria for CRh, 8 (73%) patients were previously classified as CRi and 3 (27%) as MLFS. Among 69 pts achieving CR, the median OS was 24.5 months (mo) and median EFS was 18.0 mo. CRh was achieved with a median of 1 (range 1-3) cycles. Median OS of CRh as compared to CRi was 16.9 vs 5.8 mo (HR 0.47, 95% CI 0.19-1.13, p=.06) and CRh vs MLFS was 16.9 vs 6.2 mo (HR 0.34, 95% CI 0.13-0.93, p=.007). Median EFS of pts with CRh vs CRi was 14.1 vs 5.8 mo (HR 0.49, 95%CI 0.21-1.14, p=.08) and CRh vs MLFS was 14.1 vs 6.2 mo (HR 0.37, 95% CI 0.14-1.01, p=.016). Pts achieving CRh defined a group with durable remission with duration of response of 6.0 vs 2.5 mo with CRi and 1 mo with MLFS. Additional analyses are ongoing and will be presented.
Conclusion
In pts with newly diagnosed AML and secondary AML treated with DEC10-VEN, pts achieving CRh had a trend towards improved OS and EFS compared to pts with CRi or MLFS. These findings support the use of CRh as an endpoint demonstrating clinical benefit for patients receiving venetoclax-based therapy. Larger analyses are warranted.
Keyword(s): Acute myeloid leukemia, Decitabine