
Contributions
Abstract: EP465
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Adding ENA to AZA in vitro enhances leukemic apoptosis vs AZA alone. ENA suppresses the oncometabolite, 2-hydroxyglutarate (2-HG), restoring function to α-ketoglutarate-dependent enzymes. In the AG221-AML-005 trial, combination ENA+AZA significantly improved overall response and complete remission (CR) rates vs AZA alone in patients (pts) with mutant-IDH2 (mIDH2) ND-AML not eligible for intensive chemotherapy. At an August 2019 data cutoff, median overall survival (OS) was impressive at 22 mo in both arms; OS in the AZA only arm was far longer than previously reported for AZA in ND-AML (~10-14 mo).
Aims
Assess longitudinal changes in 2-HG and IDH2 variant allele frequency (VAF) and their correlations with response, and update survival outcomes for pts in AG221-AML-005.
Methods
Pts were randomized 2:1 to ENA+AZA or AZA only. Pts received SC AZA 75mg/m2/day (d) ×7d ± ENA 100mg PO QD (continuous) in repeated 28d cycles (C). Blood 2-HG was assessed by HPLC, and BMMC mIDH2 VAF by digital PCR (LLQ 0.02–0.04%). Changes from baseline (BL) in 2-HG and IDH2 VAF were assessed in pt subgroups defined by response: CR, incomplete response (IR; CRi/CRp, PR, MLFS), or no response (NR). Updated event-free survival (EFS) and OS were assessed in August 2020. EFS was the time from randomization to relapse, PD, or death, and OS was the time from randomization to death.
Results
101 pts were randomized to ENA+AZA (n=68) or AZA only (n=33). Median age was 75 yrs (57-85). At BL, median 2-HG concentration was similar between treatment (Tx) arms (P=0.97). Median maximum 2-HG reduction from BL was –98% with ENA+AZA vs –54% with AZA (P<0.01). Median 2-HG reductions from BL with ENA+AZA were not significantly different among pts who achieved CR (–100%; n=37), IR (–98%; n=12), or NR (–93%; n=14), but in the AZA arm, median 2-HG reductions were significantly greater for pts who achieved CR (–79%; n=4) or IR (–85%; n=8) vs NR (–28%; n=17).
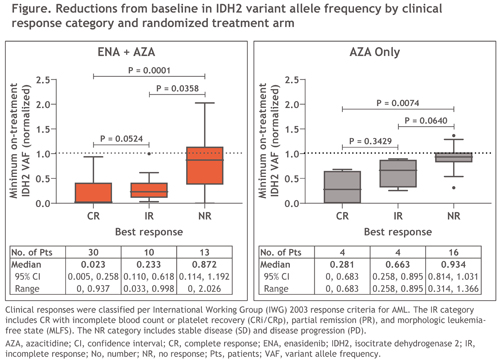
Median BL IDH2 VAF was 28% in the ENA+AZA arm and 34% in the AZA arm (P=0.06). IDH2 VAF was significantly reduced from BL in the ENA+AZA arm by C3 (–23%; P≤0.01) and continued to decrease through C11 (–98%; P≤0.001); maximal median change from BL was significantly greater with ENA+AZA vs AZA (–83.4% vs –17.7%; P<0.01). In the ENA+AZA arm, median IDH2 VAF reductions were significantly greater for pts who achieved CR (–98%; n=30) or IR (–77%; n=10) vs NR (–13%; n=13), and in the AZA arm, for pts who achieved CR (–72%; n=4) vs NR (–7%; n=16) (Figure). 10 pts achieved IDH2 molecular clearance, 9 of whom achieved CR (ENA+AZA n=8).
In August 2020, median ENA+AZA exposure was 12.1 mo and AZA exposure was 6.2 mo; 12 ENA+AZA pts remained on Tx. During follow-up, 24% of pts (16/68) in the ENA+AZA arm and 58% (19/33) in the AZA arm received subsequent AML Tx, including 11 pts in the AZA only arm (33%) who received commercially available ENA. Median EFS was nominally longer with ENA+AZA vs AZA (15.7 vs 11.9 mo; HR 0.59 [95%CI 0.31, 1.12]; P=0.10). Median OS was 22.0 mo with ENA+AZA vs 18.6 mo with AZA (HR 0.78 [0.46, 1.33]; P=0.36); 2-yr survival rates were 48.3% vs 39.9%.
Conclusion
Reductions in 2-HG and IDH2 VAF were significantly greater with ENA+AZA vs AZA only. 2-HG changes with ENA+AZA were not correlated with response but changes in IDH2 VAF were greater in responding pts. Survival was promising in both arms, and may be confounded by the high rate of subsequent AML Tx, including commercial use of ENA, in the AZA only arm during survival follow-up. Approximately one-half of pts treated with ENA+AZA were alive at 2 yrs.
Keyword(s): Acute myeloid leukemia, AML, Azacitidine, Enasidenib
Abstract: EP465
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Adding ENA to AZA in vitro enhances leukemic apoptosis vs AZA alone. ENA suppresses the oncometabolite, 2-hydroxyglutarate (2-HG), restoring function to α-ketoglutarate-dependent enzymes. In the AG221-AML-005 trial, combination ENA+AZA significantly improved overall response and complete remission (CR) rates vs AZA alone in patients (pts) with mutant-IDH2 (mIDH2) ND-AML not eligible for intensive chemotherapy. At an August 2019 data cutoff, median overall survival (OS) was impressive at 22 mo in both arms; OS in the AZA only arm was far longer than previously reported for AZA in ND-AML (~10-14 mo).
Aims
Assess longitudinal changes in 2-HG and IDH2 variant allele frequency (VAF) and their correlations with response, and update survival outcomes for pts in AG221-AML-005.
Methods
Pts were randomized 2:1 to ENA+AZA or AZA only. Pts received SC AZA 75mg/m2/day (d) ×7d ± ENA 100mg PO QD (continuous) in repeated 28d cycles (C). Blood 2-HG was assessed by HPLC, and BMMC mIDH2 VAF by digital PCR (LLQ 0.02–0.04%). Changes from baseline (BL) in 2-HG and IDH2 VAF were assessed in pt subgroups defined by response: CR, incomplete response (IR; CRi/CRp, PR, MLFS), or no response (NR). Updated event-free survival (EFS) and OS were assessed in August 2020. EFS was the time from randomization to relapse, PD, or death, and OS was the time from randomization to death.
Results
101 pts were randomized to ENA+AZA (n=68) or AZA only (n=33). Median age was 75 yrs (57-85). At BL, median 2-HG concentration was similar between treatment (Tx) arms (P=0.97). Median maximum 2-HG reduction from BL was –98% with ENA+AZA vs –54% with AZA (P<0.01). Median 2-HG reductions from BL with ENA+AZA were not significantly different among pts who achieved CR (–100%; n=37), IR (–98%; n=12), or NR (–93%; n=14), but in the AZA arm, median 2-HG reductions were significantly greater for pts who achieved CR (–79%; n=4) or IR (–85%; n=8) vs NR (–28%; n=17).
Median BL IDH2 VAF was 28% in the ENA+AZA arm and 34% in the AZA arm (P=0.06). IDH2 VAF was significantly reduced from BL in the ENA+AZA arm by C3 (–23%; P≤0.01) and continued to decrease through C11 (–98%; P≤0.001); maximal median change from BL was significantly greater with ENA+AZA vs AZA (–83.4% vs –17.7%; P<0.01). In the ENA+AZA arm, median IDH2 VAF reductions were significantly greater for pts who achieved CR (–98%; n=30) or IR (–77%; n=10) vs NR (–13%; n=13), and in the AZA arm, for pts who achieved CR (–72%; n=4) vs NR (–7%; n=16) (Figure). 10 pts achieved IDH2 molecular clearance, 9 of whom achieved CR (ENA+AZA n=8).
In August 2020, median ENA+AZA exposure was 12.1 mo and AZA exposure was 6.2 mo; 12 ENA+AZA pts remained on Tx. During follow-up, 24% of pts (16/68) in the ENA+AZA arm and 58% (19/33) in the AZA arm received subsequent AML Tx, including 11 pts in the AZA only arm (33%) who received commercially available ENA. Median EFS was nominally longer with ENA+AZA vs AZA (15.7 vs 11.9 mo; HR 0.59 [95%CI 0.31, 1.12]; P=0.10). Median OS was 22.0 mo with ENA+AZA vs 18.6 mo with AZA (HR 0.78 [0.46, 1.33]; P=0.36); 2-yr survival rates were 48.3% vs 39.9%.

Conclusion
Reductions in 2-HG and IDH2 VAF were significantly greater with ENA+AZA vs AZA only. 2-HG changes with ENA+AZA were not correlated with response but changes in IDH2 VAF were greater in responding pts. Survival was promising in both arms, and may be confounded by the high rate of subsequent AML Tx, including commercial use of ENA, in the AZA only arm during survival follow-up. Approximately one-half of pts treated with ENA+AZA were alive at 2 yrs.
Keyword(s): Acute myeloid leukemia, AML, Azacitidine, Enasidenib


