
Contributions
Abstract: EP464
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
First-generation FLT3i’s (sorafenib, midostaurin) combined with low-intensity chemotherapy (LIC) failed to improve outcomes (median overall survival (OS) ~8 months in frontline) in intensive chemotherapy (IC) non-eligible older patients (pts) with acute myeloid leukemia (AML) [Ohanian AJH 2018, Gallogly Blood 2017]. The expected median OS in FLT3-ITD mutated (m) pts with a frontline hypomethylating agent (HMA) with venetoclax (VEN) was 11.5 months [Konopleva Blood 2020]. Clinical trials exploring triplet combinations (HMA+VEN+ FLT3i) are ongoing.
Aims
We retrospectively analyzed IC non-eligible older pts with newly diagnosed FLT3m AML treated with HMA + VEN + FLT3i (triplet regimen) at MDACC between 2012-2020 and compared CR/CRi rates, measurable residual disease (MRD) dynamics, and OS with pts who received LIC + FLT3i (doublet regimen).
Methods
We identified 77 adult pts with newly diagnosed FLT3m (ITD and/or TKD) AML who were treated with LIC including first-generation (sorafenib, midostaurin) LIC doublet (n=44), second-generation (quizartinib) LIC doublet (n=16), and HMA-VEN-FLT3i (sorafenib, gilteritinib, midostaurin) triplet (n=17). Measurable residual disease (MRD) assessments were done at initial CR/CRi and longitudinally on therapy by multiplex PCR for ITD and D835, sensitivity 10-3 (Luthra, 2014, Haematologica), and by multicolor flow-cytometry (MFC), sensitivity 10-4 (Jaso, 2014, BMT).
Results
The median age was 71 [51-83], 71 [64-83], and 69 [40-80] year-old among first-generation LIC doublet, second-generation LIC doublet, and HMA-VEN-FLT3i triplet groups.
Among 77 pts treated with LIC (60 doublets, 17 triplets), 74% (57/77) achieved CR/CRi with the best PCR and MFC MRD-negativity rates of 67% (36/54) and 56% (31/55). Comparing pts treated with second-generation (n=16) versus first-generation (n=44) FLT3i based LIC doublets we noted increased CR/CRi (88% vs 64%, P=0.07) but no statistically significant difference in best FLT3 PCR (62% vs 50%, p=0.5) and best MFC MRD (50% vs 42%, p=0.6) clearance. Pts who received triplet regimens (n=17) achieved a 88% CR/CRi (similar to second-generation doublet), but with a significantly higher best FLT3 PCR (100% vs 54%, p=0.001) and higher best MFC MRD (87% vs 45%, p=0.006) clearance, compared with all patients treated with LIC doublets (n=60).
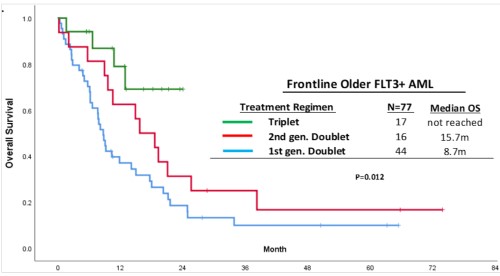
With a median follow-up of 28 months, pts treated with triplets achieved the best median OS compared with pts treated with first- or second-generation FLT3i based LIC doublets (not reached vs 15.7 vs 8.7 months (m), respectively, p=0.01) [Figure].
Across the 55 evaluable LIC patients, the achievement of MRD negativity by MFC at any time on therapy was associated with improved med OS (21m vs 12m, p=0.04. Achieving FLT3 PCR clearance at any time on therapy was associated with a non-statistically significant improved med OS (21m vs 9m, p=0.18). Pts who achieved both PCR and MFC vs MFC or PCR only clearance vs clearance of neither at any time on therapy had med OS of 21m, 20m, and 9m, resp (p=0.06).
Conclusion
The outcomes in older patients who received LIC+FLT3i (doublets) are poor, although combining LIC with second-generation inhibitors such as quizartinib appeared to improve OS. Triplet combination of HMA-VEN-FLT3i appeared to be most effective in improving OS, FLT3 PCR, and MFC clearance. Achievement of MRD negativity (especially MFC negativity) significantly improved OS, irrespective of LIC or IC backbones, and may be a surrogate endpoint to consider for future FLT3 frontline trials.
Keyword(s): AML, Flt3 inhibitor
Abstract: EP464
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
First-generation FLT3i’s (sorafenib, midostaurin) combined with low-intensity chemotherapy (LIC) failed to improve outcomes (median overall survival (OS) ~8 months in frontline) in intensive chemotherapy (IC) non-eligible older patients (pts) with acute myeloid leukemia (AML) [Ohanian AJH 2018, Gallogly Blood 2017]. The expected median OS in FLT3-ITD mutated (m) pts with a frontline hypomethylating agent (HMA) with venetoclax (VEN) was 11.5 months [Konopleva Blood 2020]. Clinical trials exploring triplet combinations (HMA+VEN+ FLT3i) are ongoing.
Aims
We retrospectively analyzed IC non-eligible older pts with newly diagnosed FLT3m AML treated with HMA + VEN + FLT3i (triplet regimen) at MDACC between 2012-2020 and compared CR/CRi rates, measurable residual disease (MRD) dynamics, and OS with pts who received LIC + FLT3i (doublet regimen).
Methods
We identified 77 adult pts with newly diagnosed FLT3m (ITD and/or TKD) AML who were treated with LIC including first-generation (sorafenib, midostaurin) LIC doublet (n=44), second-generation (quizartinib) LIC doublet (n=16), and HMA-VEN-FLT3i (sorafenib, gilteritinib, midostaurin) triplet (n=17). Measurable residual disease (MRD) assessments were done at initial CR/CRi and longitudinally on therapy by multiplex PCR for ITD and D835, sensitivity 10-3 (Luthra, 2014, Haematologica), and by multicolor flow-cytometry (MFC), sensitivity 10-4 (Jaso, 2014, BMT).
Results
The median age was 71 [51-83], 71 [64-83], and 69 [40-80] year-old among first-generation LIC doublet, second-generation LIC doublet, and HMA-VEN-FLT3i triplet groups.
Among 77 pts treated with LIC (60 doublets, 17 triplets), 74% (57/77) achieved CR/CRi with the best PCR and MFC MRD-negativity rates of 67% (36/54) and 56% (31/55). Comparing pts treated with second-generation (n=16) versus first-generation (n=44) FLT3i based LIC doublets we noted increased CR/CRi (88% vs 64%, P=0.07) but no statistically significant difference in best FLT3 PCR (62% vs 50%, p=0.5) and best MFC MRD (50% vs 42%, p=0.6) clearance. Pts who received triplet regimens (n=17) achieved a 88% CR/CRi (similar to second-generation doublet), but with a significantly higher best FLT3 PCR (100% vs 54%, p=0.001) and higher best MFC MRD (87% vs 45%, p=0.006) clearance, compared with all patients treated with LIC doublets (n=60).
With a median follow-up of 28 months, pts treated with triplets achieved the best median OS compared with pts treated with first- or second-generation FLT3i based LIC doublets (not reached vs 15.7 vs 8.7 months (m), respectively, p=0.01) [Figure].
Across the 55 evaluable LIC patients, the achievement of MRD negativity by MFC at any time on therapy was associated with improved med OS (21m vs 12m, p=0.04. Achieving FLT3 PCR clearance at any time on therapy was associated with a non-statistically significant improved med OS (21m vs 9m, p=0.18). Pts who achieved both PCR and MFC vs MFC or PCR only clearance vs clearance of neither at any time on therapy had med OS of 21m, 20m, and 9m, resp (p=0.06).

Conclusion
The outcomes in older patients who received LIC+FLT3i (doublets) are poor, although combining LIC with second-generation inhibitors such as quizartinib appeared to improve OS. Triplet combination of HMA-VEN-FLT3i appeared to be most effective in improving OS, FLT3 PCR, and MFC clearance. Achievement of MRD negativity (especially MFC negativity) significantly improved OS, irrespective of LIC or IC backbones, and may be a surrogate endpoint to consider for future FLT3 frontline trials.
Keyword(s): AML, Flt3 inhibitor


