
Contributions
Abstract: EP462
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Cytarabine, the backbone of acute myeloid leukemia (AML) standard of care chemotherapy, is associated with a toxicity which precludes its administration to older patients and those with comorbidities. Aspacytarabine (BST-236) is a prodrug of cytarabine. Its pharmacokinetics and metabolism lead to gradual release of cytarabine with shorter duration at peak toxic levels compared to direct cytarabine administration, resulting in relative sparing of normal tissues and enabling delivery of high cytarabine doses to patients otherwise unfit to receive it.
Aims
To evaluate the efficacy and safety of aspacytarabine as a first-line, single-agent therapy in newly-diagnosed AML patients unfit for standard induction therapy.
Methods
Aspacytarabine is administrated at a dose of 4.5 g/m2/d (equimolar of 3 g/m2/d cytarabine). Therapy is time-limited, with only 1-2 induction courses and 1-3 consolidation courses, each consists of 6 daily 1-hour intravenous infusions. Newly-diagnosed AML patients unfit for standard chemotherapy, including those with secondary AML, previously treated with HMA, and patients with therapy-related AML, are eligible.
Results
To date, 71 AML patients were treated with aspacytarabine; 47 newly-diagnosed AML patients unfit for standard chemotherapy (median age 75 years) completed 1-4 courses of 4.5 g/m2/d aspacytarabine in an ongoing phase 2b study, including 30 patients (64%) with de novo AML and 17 (36%) with secondary AML. Six patients (13%) were previously treated with HMA (median 12 courses), and 21 (45%) patients had ECOG Performance Status 2. The median baseline bone marrow blast percentage was 45 (range 13-94), and 54% and 29% of patients had adverse or intermediate ELN score, respectively.
Aspacytarabine is safe and well-tolerated in repeated-course administration. Grade >2 drug-related adverse events include mainly hematological events and infections. The 30-day mortality rate is 11%.
Of the 43 phase 2b patients evaluable for efficacy analysis to date, 15 patients (35%) reached a complete remission (CR) following 1 (13 patients) or 2 (2 patients) aspacytarabine induction courses. All responders reached a complete hematological recovery (median 27.5 days, range 22-39 days). The CR rate excluding patients with prior HMA therapy is 41%, and 46% in de novo AML patients. The CR rate in patients at the age of ≥75 years and patients with adverse ELN score is 33%.
Of note, of the 11 patients evaluable to date for minimal residual disease (MRD) analysis, 8 (73%) are MRD negative.
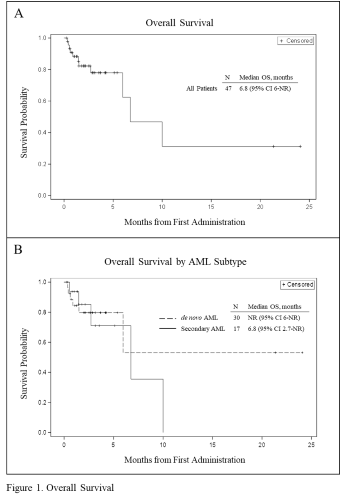
While aspacytarabine treatment consists of a limited number of courses, median duration of response (DOR) and median overall survival (OS) for responders are not reached (NR) at end of follow up, 12 (95% CI 5-NR) and 24 (95% CI 6-NR) months, respectively. The median OS for all patients is 6.8 (95% CI 6-NR) months (Figure 1A). Median OS for de novo AML patients is NR (95% CI 6-NR) and 6.8 (95% CI 2.7-NR) for secondary AML patients (Figure 1B).
Follow-up is ongoing with additional patients enrolling on study; updated analysis will be presented at the meeting.
Conclusion
The cumulative clinical data suggest that aspacytarabine monotherapy is safe and efficacious as a first-line therapy for patients who are unfit for intensive chemotherapy, including patients with adverse cytogenetics and patients ≥75 year of age. The data may establish aspacytarabine, already granted Fast Track designation from the FDA, as a new intensive therapy backbone of AML and may, for the first time, allow older adults benefit from standard intensive therapy.
Keyword(s): Acute myeloid leukemia, Chemotherapy, Elderly, High dose therapy
Abstract: EP462
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Cytarabine, the backbone of acute myeloid leukemia (AML) standard of care chemotherapy, is associated with a toxicity which precludes its administration to older patients and those with comorbidities. Aspacytarabine (BST-236) is a prodrug of cytarabine. Its pharmacokinetics and metabolism lead to gradual release of cytarabine with shorter duration at peak toxic levels compared to direct cytarabine administration, resulting in relative sparing of normal tissues and enabling delivery of high cytarabine doses to patients otherwise unfit to receive it.
Aims
To evaluate the efficacy and safety of aspacytarabine as a first-line, single-agent therapy in newly-diagnosed AML patients unfit for standard induction therapy.
Methods
Aspacytarabine is administrated at a dose of 4.5 g/m2/d (equimolar of 3 g/m2/d cytarabine). Therapy is time-limited, with only 1-2 induction courses and 1-3 consolidation courses, each consists of 6 daily 1-hour intravenous infusions. Newly-diagnosed AML patients unfit for standard chemotherapy, including those with secondary AML, previously treated with HMA, and patients with therapy-related AML, are eligible.
Results
To date, 71 AML patients were treated with aspacytarabine; 47 newly-diagnosed AML patients unfit for standard chemotherapy (median age 75 years) completed 1-4 courses of 4.5 g/m2/d aspacytarabine in an ongoing phase 2b study, including 30 patients (64%) with de novo AML and 17 (36%) with secondary AML. Six patients (13%) were previously treated with HMA (median 12 courses), and 21 (45%) patients had ECOG Performance Status 2. The median baseline bone marrow blast percentage was 45 (range 13-94), and 54% and 29% of patients had adverse or intermediate ELN score, respectively.
Aspacytarabine is safe and well-tolerated in repeated-course administration. Grade >2 drug-related adverse events include mainly hematological events and infections. The 30-day mortality rate is 11%.
Of the 43 phase 2b patients evaluable for efficacy analysis to date, 15 patients (35%) reached a complete remission (CR) following 1 (13 patients) or 2 (2 patients) aspacytarabine induction courses. All responders reached a complete hematological recovery (median 27.5 days, range 22-39 days). The CR rate excluding patients with prior HMA therapy is 41%, and 46% in de novo AML patients. The CR rate in patients at the age of ≥75 years and patients with adverse ELN score is 33%.
Of note, of the 11 patients evaluable to date for minimal residual disease (MRD) analysis, 8 (73%) are MRD negative.
While aspacytarabine treatment consists of a limited number of courses, median duration of response (DOR) and median overall survival (OS) for responders are not reached (NR) at end of follow up, 12 (95% CI 5-NR) and 24 (95% CI 6-NR) months, respectively. The median OS for all patients is 6.8 (95% CI 6-NR) months (Figure 1A). Median OS for de novo AML patients is NR (95% CI 6-NR) and 6.8 (95% CI 2.7-NR) for secondary AML patients (Figure 1B).
Follow-up is ongoing with additional patients enrolling on study; updated analysis will be presented at the meeting.

Conclusion
The cumulative clinical data suggest that aspacytarabine monotherapy is safe and efficacious as a first-line therapy for patients who are unfit for intensive chemotherapy, including patients with adverse cytogenetics and patients ≥75 year of age. The data may establish aspacytarabine, already granted Fast Track designation from the FDA, as a new intensive therapy backbone of AML and may, for the first time, allow older adults benefit from standard intensive therapy.
Keyword(s): Acute myeloid leukemia, Chemotherapy, Elderly, High dose therapy


