
Contributions
Abstract: EP460
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Cytogenetically normal acute myeloid leukemia (CN-AML) patients with biallelic CEBPA (biCEBPA) mutations have a favorable prognosis compared with patients with wild-type or monoallelically mutated CEBPA. However, this subgroup is still not homogeneous with relapse rate reaching approximately 40% and the best post-remission therapy remains unclear.
Aims
Elucidate the cooperating events in CN-AML with biCEBPA mutations, identify the risk factors and explore the optimal post-remission therapy in different risk subgroups.
Methods
A total of 158 CN-AML patients with biCEBPA mutations were enrolled for targeted region sequencing by a panel with 236 genes. Patients were assigned to a training cohort (N =111) and a validation cohort (N =47) at a ratio of 7:3 randomly to construct a nomogram model. Risk factors identified in nomogram were incorporated to stratify the cohort. Post-remission therapies were compared in different risk subgroups. Receiving an allo-HSCT was recorded as a censored event to identify the prognostic factors before an allo-HSCT. Landmark analysis was performed to revise bias from early relapse or death when comparing the outcomes of post-remission therapies.
Results
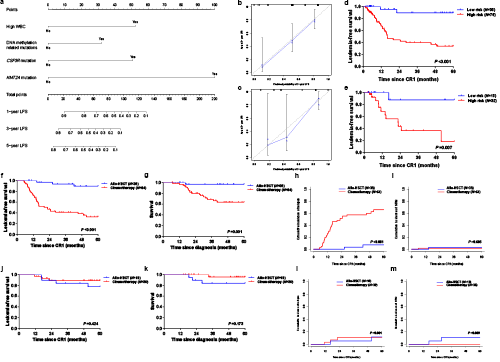
We identified 1,306 mutations in 203 genes other than CEBPA. The median mutation number was 8 (1–20). Patients with low mutational context (mutation number <8) showed significantly higher 5-year leukemia-free survival (LFS) (61.6% vs. 39.0%, P =0.033) and 5-year survival (85.6% vs. 62.9%, P =0.030), lower 5-year cumulative incidence of relapse (CIR) (38.4% vs. 59.5%, P =0.0496) and comparable 5-year non-relapse mortality (NRM) (0 vs. 1.5%, P =0.407). Four variables were in the nomogram model: WBC (high vs. low, represents >18.30×109/L vs. ≤18.30×109/L), CSF3R mutation (+ vs. -), KMT2A mutation (+ vs. -) and DNA methylation related mutation (mutations in TET2, DNMT3A, IDH2, BAZ2A and IDH1) (Figure 1a). The C-index was 0.750 (95% CI, 0.670–0.830) in training cohort and 0.771 (95% CI, 0.661–0.881) in the validation with good calibration in the two cohorts (Figure 1b-c). According to the nomogram model, patients with none of the risk factors were classified as the low-risk subgroup (N =50) and the remain were high-risk (N =108). Low-risk patients had significantly lower positive MRD after induction (25.5% vs. 57.7%, P <0.001) and better prognosis both in training and validation cohorts (5-year LFS, 89.3% vs. 33.8%, P <0.001; 87.5% vs. 18.2%, P =0.009) (Figure 1d-e). Post-remission therapies analyses indicated that allo-HSCT had better 5-year LFS (89.6% vs. 32.6%, P <0.001), 5-year survival (96.9% vs. 63.6%, P =0.001) and lower 5-year CIR (7.2% vs. 65.8%, P <0.001) compared with consolidation chemotherapy in high-risk subgroup (Figure 1f-h). However, in low-risk subgroup, these two post-remission therapies did not show significant difference (allo-HSCT vs. consolidation chemotherapy, 5-year LFS, 77.4% vs. 88.9%, P =0.424; 5-year survival, 83.9% vs. 95.5%, P =0.173; 5-year CIR, 11.7% vs. 11.1%, P =0.901) (Figure 1j-l). The 5-year NRM was comparable in high- (3.1% vs. 1.6%, P =0.685) (Figure 1i) and low-risk (10.9% vs. 0, P =0.099) (Figure 1m) subgroups.
Conclusion
CN-AML patients with biCEBPA mutations were prognostically heterogeneous with different mutational context. Only approximately one third of these patients represented the low-risk subgroup, and consolidation chemotherapy should be given as the first line post-remission therapy. While in the high-risk subgroup, allo-HSCT is recommended.
Keyword(s): Acute myeloid leukemia, Allogeneic hematopoietic stem cell transplant, CCAAT/enhancer binding protein alpha (C/EBPa), Prognostic groups
Abstract: EP460
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Cytogenetically normal acute myeloid leukemia (CN-AML) patients with biallelic CEBPA (biCEBPA) mutations have a favorable prognosis compared with patients with wild-type or monoallelically mutated CEBPA. However, this subgroup is still not homogeneous with relapse rate reaching approximately 40% and the best post-remission therapy remains unclear.
Aims
Elucidate the cooperating events in CN-AML with biCEBPA mutations, identify the risk factors and explore the optimal post-remission therapy in different risk subgroups.
Methods
A total of 158 CN-AML patients with biCEBPA mutations were enrolled for targeted region sequencing by a panel with 236 genes. Patients were assigned to a training cohort (N =111) and a validation cohort (N =47) at a ratio of 7:3 randomly to construct a nomogram model. Risk factors identified in nomogram were incorporated to stratify the cohort. Post-remission therapies were compared in different risk subgroups. Receiving an allo-HSCT was recorded as a censored event to identify the prognostic factors before an allo-HSCT. Landmark analysis was performed to revise bias from early relapse or death when comparing the outcomes of post-remission therapies.
Results
We identified 1,306 mutations in 203 genes other than CEBPA. The median mutation number was 8 (1–20). Patients with low mutational context (mutation number <8) showed significantly higher 5-year leukemia-free survival (LFS) (61.6% vs. 39.0%, P =0.033) and 5-year survival (85.6% vs. 62.9%, P =0.030), lower 5-year cumulative incidence of relapse (CIR) (38.4% vs. 59.5%, P =0.0496) and comparable 5-year non-relapse mortality (NRM) (0 vs. 1.5%, P =0.407). Four variables were in the nomogram model: WBC (high vs. low, represents >18.30×109/L vs. ≤18.30×109/L), CSF3R mutation (+ vs. -), KMT2A mutation (+ vs. -) and DNA methylation related mutation (mutations in TET2, DNMT3A, IDH2, BAZ2A and IDH1) (Figure 1a). The C-index was 0.750 (95% CI, 0.670–0.830) in training cohort and 0.771 (95% CI, 0.661–0.881) in the validation with good calibration in the two cohorts (Figure 1b-c). According to the nomogram model, patients with none of the risk factors were classified as the low-risk subgroup (N =50) and the remain were high-risk (N =108). Low-risk patients had significantly lower positive MRD after induction (25.5% vs. 57.7%, P <0.001) and better prognosis both in training and validation cohorts (5-year LFS, 89.3% vs. 33.8%, P <0.001; 87.5% vs. 18.2%, P =0.009) (Figure 1d-e). Post-remission therapies analyses indicated that allo-HSCT had better 5-year LFS (89.6% vs. 32.6%, P <0.001), 5-year survival (96.9% vs. 63.6%, P =0.001) and lower 5-year CIR (7.2% vs. 65.8%, P <0.001) compared with consolidation chemotherapy in high-risk subgroup (Figure 1f-h). However, in low-risk subgroup, these two post-remission therapies did not show significant difference (allo-HSCT vs. consolidation chemotherapy, 5-year LFS, 77.4% vs. 88.9%, P =0.424; 5-year survival, 83.9% vs. 95.5%, P =0.173; 5-year CIR, 11.7% vs. 11.1%, P =0.901) (Figure 1j-l). The 5-year NRM was comparable in high- (3.1% vs. 1.6%, P =0.685) (Figure 1i) and low-risk (10.9% vs. 0, P =0.099) (Figure 1m) subgroups.

Conclusion
CN-AML patients with biCEBPA mutations were prognostically heterogeneous with different mutational context. Only approximately one third of these patients represented the low-risk subgroup, and consolidation chemotherapy should be given as the first line post-remission therapy. While in the high-risk subgroup, allo-HSCT is recommended.
Keyword(s): Acute myeloid leukemia, Allogeneic hematopoietic stem cell transplant, CCAAT/enhancer binding protein alpha (C/EBPa), Prognostic groups


