
Contributions
Abstract: EP458
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Patients with acute myeloid leukemia (AML) and persistent measurable residual disease (MRD) have a poor prognosis and new treatment modalities for prevention of relapse i.e. by conversion of MRD status are being investigated. In a phase I study it was shown that the allogeneic leukemia-derived dendritic cell vaccine, DCP-001, induces both humoral and cellular immune responses (van de Loosdrecht et al., Cancer Immunol. Immunother. 2018).
Aims
A phase II trial is currently conducted to investigate the ability of DCP-001 to generate an anti-leukemia response in patients with AML in CR but MRD positive and to convert patients to from MRD positive to a MRD negative status (Clinicaltrials.gov: NCT03697707). Immunological analyses are planned to elucidate the height and breadth of the vaccination on the local and systemic immune responses before, during and after the vaccination regimen.
Methods
An intended total of 20 patients (10 at 25e6 and 10 at 50e6 dose) ineligible for HSCT will receive a primary regimen consisting of 4 doses given biweekly (week 0, 2, 4 and 6) followed by the booster regimen with 2 doses (10e6) at week 14 and 18. MRD status (flow cytometry and/or qPCR based according to ELN guidelines) is assessed at baseline, before booster vaccination (week 14) as well as 2 weeks and 3 months after the booster to monitor clinical efficacy of the vaccinations. At present (March 1, 2021), the 25e6 dose cohort has been fully enrolled and most patients have completed the 32 week end of treatment visit, while the 50e6 cohort has been nearly completed with 7 patients having received at least one vaccination.
Results
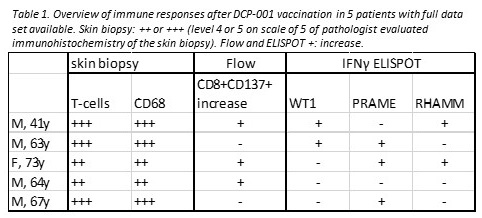
First analyses of skin biopsies taken 2 days after the 1st, 4th and 6th dose of patients whom have completed the full vaccination schedule showed acute inflammation in the skin reflected by induration and redness of the skin. Infiltrating immune cells consisted mainly of CD4 and CD8 T-cells as well as CD68+ cells .
Flow cytometric analysis on PBMC showed an increase in antigen experienced CD8+ CD137+ T-cells already 2 days after vaccination. After the primary schedule, immune responses to known AML tumor-associated antigens such as PRAME and WT-1 were induced post- DCP-001 vaccination as measured by IFNy ELISPOT. Patients showed multiple detectable responses, either boosted from preexisting or as an induced primary response upon DCP-001 vaccination. Up to a 4-fold median increase to baseline levels after initial vaccinations was detected, in patients with positive ELISPOT responses. After primary vaccinations, retraction of the cellular responses was observed, but could be boosted, with a 1.5 fold median increase compared to pre-boosting levels.
Consecutive samples of 3 patients were analyzed by TCRbeta sequencing and showed increased breadth and height of T-cell clones after DCP-001 vaccination. As DCP-001 is known to induce responses to tumor associated antigens, not only expressed by DCP-001, such a NY-ESO-1, a broad range of different TCRs to several published TAA was detected.
At time of reporting, 2 of 5 patients with immune response data available, MRD negativity was achieved. In both patients multiple antigenic responses after DCP-001 vaccination and high levels of immune cells infiltrates at the injection site were observed.
Conclusion
DCP-001 used as a relapse vaccine in AML patients, showed recruitment of immune cells to the skin, increased cellular responses to a broad range of tumor associated antigens, and resulted in reversion of the MRD status in some patients.
Keyword(s): AML, MRD, T cell response, Vaccination
Abstract: EP458
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Patients with acute myeloid leukemia (AML) and persistent measurable residual disease (MRD) have a poor prognosis and new treatment modalities for prevention of relapse i.e. by conversion of MRD status are being investigated. In a phase I study it was shown that the allogeneic leukemia-derived dendritic cell vaccine, DCP-001, induces both humoral and cellular immune responses (van de Loosdrecht et al., Cancer Immunol. Immunother. 2018).
Aims
A phase II trial is currently conducted to investigate the ability of DCP-001 to generate an anti-leukemia response in patients with AML in CR but MRD positive and to convert patients to from MRD positive to a MRD negative status (Clinicaltrials.gov: NCT03697707). Immunological analyses are planned to elucidate the height and breadth of the vaccination on the local and systemic immune responses before, during and after the vaccination regimen.
Methods
An intended total of 20 patients (10 at 25e6 and 10 at 50e6 dose) ineligible for HSCT will receive a primary regimen consisting of 4 doses given biweekly (week 0, 2, 4 and 6) followed by the booster regimen with 2 doses (10e6) at week 14 and 18. MRD status (flow cytometry and/or qPCR based according to ELN guidelines) is assessed at baseline, before booster vaccination (week 14) as well as 2 weeks and 3 months after the booster to monitor clinical efficacy of the vaccinations. At present (March 1, 2021), the 25e6 dose cohort has been fully enrolled and most patients have completed the 32 week end of treatment visit, while the 50e6 cohort has been nearly completed with 7 patients having received at least one vaccination.
Results
First analyses of skin biopsies taken 2 days after the 1st, 4th and 6th dose of patients whom have completed the full vaccination schedule showed acute inflammation in the skin reflected by induration and redness of the skin. Infiltrating immune cells consisted mainly of CD4 and CD8 T-cells as well as CD68+ cells .
Flow cytometric analysis on PBMC showed an increase in antigen experienced CD8+ CD137+ T-cells already 2 days after vaccination. After the primary schedule, immune responses to known AML tumor-associated antigens such as PRAME and WT-1 were induced post- DCP-001 vaccination as measured by IFNy ELISPOT. Patients showed multiple detectable responses, either boosted from preexisting or as an induced primary response upon DCP-001 vaccination. Up to a 4-fold median increase to baseline levels after initial vaccinations was detected, in patients with positive ELISPOT responses. After primary vaccinations, retraction of the cellular responses was observed, but could be boosted, with a 1.5 fold median increase compared to pre-boosting levels.
Consecutive samples of 3 patients were analyzed by TCRbeta sequencing and showed increased breadth and height of T-cell clones after DCP-001 vaccination. As DCP-001 is known to induce responses to tumor associated antigens, not only expressed by DCP-001, such a NY-ESO-1, a broad range of different TCRs to several published TAA was detected.
At time of reporting, 2 of 5 patients with immune response data available, MRD negativity was achieved. In both patients multiple antigenic responses after DCP-001 vaccination and high levels of immune cells infiltrates at the injection site were observed.

Conclusion
DCP-001 used as a relapse vaccine in AML patients, showed recruitment of immune cells to the skin, increased cellular responses to a broad range of tumor associated antigens, and resulted in reversion of the MRD status in some patients.
Keyword(s): AML, MRD, T cell response, Vaccination


