
Contributions
Abstract: EP453
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Homoharringtonine (HHT) is a natural alkaloid derivate from Cephalotaxus which has potent anti-leukemia effect in acute myeloid leukemia (AML). Azacitidine (AZA), a DNA hypomethylation agent, has been approved to treat AML. GATA2 is an essential transcription factor in hematopoietic differentiation. Mutations in GATA2 have been detected in AML and are widely recognized as to be associated with inferior clinical outcome. However, no specific treatment regimen has been tested or recommended for AML with GATA2 mutations.
Aims
This study is to investigate the synergistic anti-leukemia effect of combination of AZA and HHT in GATA2-mutated AML in a registered clinical study (ClinicalTrials.gov ID: NCT04248595) and explore the potential underlying mechanisms of AZA+HHT in GATA2-mutated AML.
Methods
U937 AML cell line was used to construct GATA2 mutated cells (GATA2A318T and GATA2A372T) and GATA2 wild type cells (GATA2WT) by lentiviral transfection. CCK-8 cell proliferation assay and CalcuSyn software were used to analyze the synergistic effect of AZA+HHT in GATA2 mutated and wild type cells in vitro. RNA sequencing (RNA-seq) was performed before and after U937 cells treated with AZA, HHT and vehicle control for 48 hours.
Results
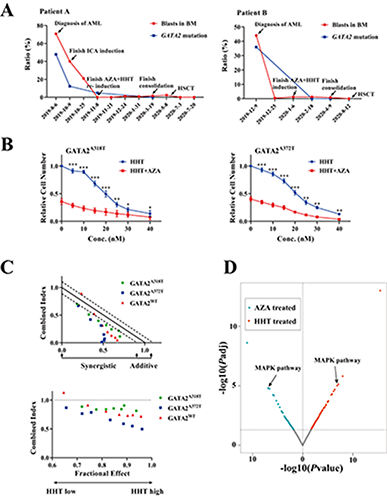
Two GATA2 mutations were identified in two AML patients. Patient A is a transformed AML (M2) with a history of granulocytic sarcomas. Flow cytometry revealed 86.38% blasts in the bone marrow at the diagnosis of AML. Whole exome sequencing (WES) showed that there was a 47.8% missense mutation in GATA2 (c.1114G>A, p.A372T). No remission was achieved after one cycle of induction therapy with ICA regimen. Then, the patient was recruited to the Clinical study and AZA+HHT based regimen was given as the re-induction therapy. A complete remission (CR) was achieved after 14 days and the GATA2 mutation ratio significantly declined to 4.82%. Allogeneic hematopoietic stem cell transplantation (allo-SCT) was performed after two cycles of high-dose cytarabine consolidation (Figure 1A). Patient B is a de novo AML (M2) with 44% blasts and 35.95% GATA2 missense mutation (c.952G>A, p.A318T) at diagnosis. The patient was recruited to the Clinical study and achieved CR with negative GATA2 mutation after AZA+HHT based regimen as the induction therapy. Two cycles of high-dose cytarabine consolidation were received followed by allo-SCT (Figure 1A). In vitro assay revealed a synergistic effect of AZA+HHT on cell proliferation arrest in GATA2A318T and GATA2A372T cells compared with single drug (P<0.05 in all concentration groups) (Figure 1B). The synergistic effect was stronger (lower combined index) in both GATA2A318T and GATA2A372T cells than GATA2WT cells in low HHT concentration groups (Figure 1B). AZA+HHT presented more potent synergistic effect in GATA2A372T cells compared with GATA2WT and GATA2A318T cells in all HHT concentration groups, suggesting that GATA2A372T was more sensitive to AZA+HHT (Figure 1C). Furthermore, RNA-seq revealed that MAPK pathway was significantly altered in both AZA and HHT treated groups (Padj<0.001), indicating that AZA+HHT exhibited strong synergistic anti-leukemia effect in GATA2-mutated AML and may rely on their common effect of targeting MAPK pathway (Figure 1D).
Conclusion
Our data for the first time showed that the synergistic efficacy of novel combination-based regimen AZA+HHT in GATA2-mutated AML. Targeting MAPK pathway may be one of the potential downstream mechanisms of AZA+HHT in treating GATA2-mutated AML.
Keyword(s): Acute myeloid leukemia, Azacitidine, GATA-2, Homoharringtonine
Abstract: EP453
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Homoharringtonine (HHT) is a natural alkaloid derivate from Cephalotaxus which has potent anti-leukemia effect in acute myeloid leukemia (AML). Azacitidine (AZA), a DNA hypomethylation agent, has been approved to treat AML. GATA2 is an essential transcription factor in hematopoietic differentiation. Mutations in GATA2 have been detected in AML and are widely recognized as to be associated with inferior clinical outcome. However, no specific treatment regimen has been tested or recommended for AML with GATA2 mutations.
Aims
This study is to investigate the synergistic anti-leukemia effect of combination of AZA and HHT in GATA2-mutated AML in a registered clinical study (ClinicalTrials.gov ID: NCT04248595) and explore the potential underlying mechanisms of AZA+HHT in GATA2-mutated AML.
Methods
U937 AML cell line was used to construct GATA2 mutated cells (GATA2A318T and GATA2A372T) and GATA2 wild type cells (GATA2WT) by lentiviral transfection. CCK-8 cell proliferation assay and CalcuSyn software were used to analyze the synergistic effect of AZA+HHT in GATA2 mutated and wild type cells in vitro. RNA sequencing (RNA-seq) was performed before and after U937 cells treated with AZA, HHT and vehicle control for 48 hours.
Results
Two GATA2 mutations were identified in two AML patients. Patient A is a transformed AML (M2) with a history of granulocytic sarcomas. Flow cytometry revealed 86.38% blasts in the bone marrow at the diagnosis of AML. Whole exome sequencing (WES) showed that there was a 47.8% missense mutation in GATA2 (c.1114G>A, p.A372T). No remission was achieved after one cycle of induction therapy with ICA regimen. Then, the patient was recruited to the Clinical study and AZA+HHT based regimen was given as the re-induction therapy. A complete remission (CR) was achieved after 14 days and the GATA2 mutation ratio significantly declined to 4.82%. Allogeneic hematopoietic stem cell transplantation (allo-SCT) was performed after two cycles of high-dose cytarabine consolidation (Figure 1A). Patient B is a de novo AML (M2) with 44% blasts and 35.95% GATA2 missense mutation (c.952G>A, p.A318T) at diagnosis. The patient was recruited to the Clinical study and achieved CR with negative GATA2 mutation after AZA+HHT based regimen as the induction therapy. Two cycles of high-dose cytarabine consolidation were received followed by allo-SCT (Figure 1A). In vitro assay revealed a synergistic effect of AZA+HHT on cell proliferation arrest in GATA2A318T and GATA2A372T cells compared with single drug (P<0.05 in all concentration groups) (Figure 1B). The synergistic effect was stronger (lower combined index) in both GATA2A318T and GATA2A372T cells than GATA2WT cells in low HHT concentration groups (Figure 1B). AZA+HHT presented more potent synergistic effect in GATA2A372T cells compared with GATA2WT and GATA2A318T cells in all HHT concentration groups, suggesting that GATA2A372T was more sensitive to AZA+HHT (Figure 1C). Furthermore, RNA-seq revealed that MAPK pathway was significantly altered in both AZA and HHT treated groups (Padj<0.001), indicating that AZA+HHT exhibited strong synergistic anti-leukemia effect in GATA2-mutated AML and may rely on their common effect of targeting MAPK pathway (Figure 1D).

Conclusion
Our data for the first time showed that the synergistic efficacy of novel combination-based regimen AZA+HHT in GATA2-mutated AML. Targeting MAPK pathway may be one of the potential downstream mechanisms of AZA+HHT in treating GATA2-mutated AML.
Keyword(s): Acute myeloid leukemia, Azacitidine, GATA-2, Homoharringtonine


