
Contributions
Abstract: EP451
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
KMT2A partial tandem duplication (PTD) has been reported in 3–10% of adult AML cases and is mutually exclusive of 11q23 rearrangements. Clinical implications of KMT2A-PTD on outcome remain unclear with conflicting results in the literature. As a consequence, if KMT2A-rearranged AML are considered of unfavorable prognosis within ELN 2017 classification, KMT2A-PTD is not included in current AML prognostic classifications, and so, not considered as an independent factor for hematopoietic stem cell transplantation (HSCT) allocation.
Aims
In this study, we aimed to evaluate KMT2A-PTD impact on outcome in newly diagnosed AML patients according to treatment intensity and HSCT.
Methods
Seventy-nine patients treated in Lyon and Bordeaux, France, with newly diagnosed KMT2A-PTD mutated AML were included in this retrospective study, between November 2005 and August 2019.
Results
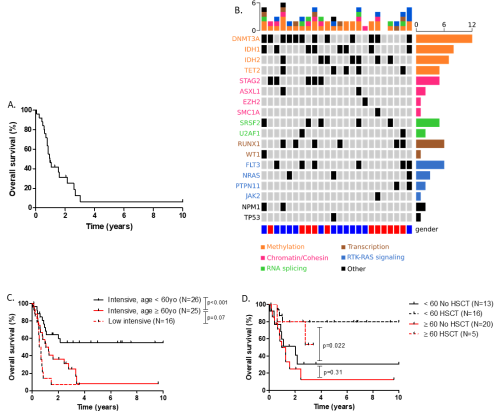
Median age of the entire cohort was 66 years old (range 19-87). Overall, most of the patients had a normal karyotype (65.3%). Regarding 2013 ELN classification, 11.4, 77.2 and 11.2% were favorable, intermediate I/II and unfavorable respectively. The median overall survival (OS) was 12.1 months with an OS rate of 50.1 % at 1 year and 30.9% at 3 years (Figure A). NGS was available for 24 intensively treated patients. The most frequently observed mutations were those affecting DNA methylation (DNMT3A, TET2, IDH1/2), found in 91% (21/23) of cases. Receptor tyrosine kinase (RTK) and RAS pathways were mutated in about 44% of patients. Other pathways commonly found altered was the chromatin/cohesin complex (ASXL1, STAG2, SMC1A, EZH2), spliceosome (U2AF1, SRFS2) and transcription factors (RUNX1, WT1) in 39%, 30% and 30% of cases respectively (figure B). Regarding the impact of co-mutations on survival, the presence of FLT3-ITD was associated with an inferior outcome (median OS: 9.36 vs 33.4 months, p=0.033). Median OS was not reached, 33.4 and 29.4 months for NPM1, IDH1/2 and DNMT3A mutated patients respectively. In contrast, median OS was shorter for patients harboring RTK (FLT3-ITD, NRAS, PTPN11) and TP53/ASXL1/RUNX1 mutations (9.6 and 11.9 months respectively).
In patients younger than 60 years old treated with intensive chemotherapy, median overall survival was not reached compared to 12.6 months in older ones (p<0.001). In contrast, there was no statistical difference in OS in elderly patients treated with intensive or low intensive regimens (Figure, C). Twenty-one patients underwent HSCT in first CR (CR1). For patients receiving HSCT at any time in CR, median OS was not reached, whatever was the age group. For those who did not underwent HSCT, median OS was 24.2 and 13.2 months in patients younger and older than 60 years old respectively, with no statistical significance between age group in these settings (Figure D). In the multivariate analysis only HSCT at any time in CR (HR=2.35; p0.034) and FLT3-ITD status (HR=0.29; p=0.014) were independent variables associated with OS.
Conclusion
This study is one of the largest KMT2A-PTD cohort emphasizing mutation impact according to treatment intensity and HSCT. In real life settings, patients harboring such mutation are characterized by a poor outcome especially in elderly. We showed that the negative impact of this mutation might be overcome by HSCT in younger patients. In elderly unfit patient, targeted therapies such as IDH inhibitors and venetoclax-based regimens might be of interest.
Keyword(s): Allogeneic hematopoietic stem cell transplant, AML, MLL
Abstract: EP451
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
KMT2A partial tandem duplication (PTD) has been reported in 3–10% of adult AML cases and is mutually exclusive of 11q23 rearrangements. Clinical implications of KMT2A-PTD on outcome remain unclear with conflicting results in the literature. As a consequence, if KMT2A-rearranged AML are considered of unfavorable prognosis within ELN 2017 classification, KMT2A-PTD is not included in current AML prognostic classifications, and so, not considered as an independent factor for hematopoietic stem cell transplantation (HSCT) allocation.
Aims
In this study, we aimed to evaluate KMT2A-PTD impact on outcome in newly diagnosed AML patients according to treatment intensity and HSCT.
Methods
Seventy-nine patients treated in Lyon and Bordeaux, France, with newly diagnosed KMT2A-PTD mutated AML were included in this retrospective study, between November 2005 and August 2019.
Results
Median age of the entire cohort was 66 years old (range 19-87). Overall, most of the patients had a normal karyotype (65.3%). Regarding 2013 ELN classification, 11.4, 77.2 and 11.2% were favorable, intermediate I/II and unfavorable respectively. The median overall survival (OS) was 12.1 months with an OS rate of 50.1 % at 1 year and 30.9% at 3 years (Figure A). NGS was available for 24 intensively treated patients. The most frequently observed mutations were those affecting DNA methylation (DNMT3A, TET2, IDH1/2), found in 91% (21/23) of cases. Receptor tyrosine kinase (RTK) and RAS pathways were mutated in about 44% of patients. Other pathways commonly found altered was the chromatin/cohesin complex (ASXL1, STAG2, SMC1A, EZH2), spliceosome (U2AF1, SRFS2) and transcription factors (RUNX1, WT1) in 39%, 30% and 30% of cases respectively (figure B). Regarding the impact of co-mutations on survival, the presence of FLT3-ITD was associated with an inferior outcome (median OS: 9.36 vs 33.4 months, p=0.033). Median OS was not reached, 33.4 and 29.4 months for NPM1, IDH1/2 and DNMT3A mutated patients respectively. In contrast, median OS was shorter for patients harboring RTK (FLT3-ITD, NRAS, PTPN11) and TP53/ASXL1/RUNX1 mutations (9.6 and 11.9 months respectively).
In patients younger than 60 years old treated with intensive chemotherapy, median overall survival was not reached compared to 12.6 months in older ones (p<0.001). In contrast, there was no statistical difference in OS in elderly patients treated with intensive or low intensive regimens (Figure, C). Twenty-one patients underwent HSCT in first CR (CR1). For patients receiving HSCT at any time in CR, median OS was not reached, whatever was the age group. For those who did not underwent HSCT, median OS was 24.2 and 13.2 months in patients younger and older than 60 years old respectively, with no statistical significance between age group in these settings (Figure D). In the multivariate analysis only HSCT at any time in CR (HR=2.35; p0.034) and FLT3-ITD status (HR=0.29; p=0.014) were independent variables associated with OS.

Conclusion
This study is one of the largest KMT2A-PTD cohort emphasizing mutation impact according to treatment intensity and HSCT. In real life settings, patients harboring such mutation are characterized by a poor outcome especially in elderly. We showed that the negative impact of this mutation might be overcome by HSCT in younger patients. In elderly unfit patient, targeted therapies such as IDH inhibitors and venetoclax-based regimens might be of interest.
Keyword(s): Allogeneic hematopoietic stem cell transplant, AML, MLL


