
Contributions
Abstract: EP448
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Gilteritinib is a FLT3 inhibitor with demonstrated efficacy and safety in patients with FLT3-mutated relapsed or refractory (R/R) AML. The phase 1/2 CHRYSALIS trial demonstrated the safety and antileukemic activity of gilteritinib in a FLT3-mutation–enriched R/R AML population (Perl AE, et al. Lancet Oncol. 2017). The phase 3 ADMIRAL trial demonstrated the superiority of gilteritinib to salvage chemotherapy (SC) in FLT3-mutated patients based on longer median overall survival (OS) with gilteritinib (9.3 vs 5.6 months; hazard ratio [HR]=0.64 [95% CI: 0.49, 0.83]; P<0.001) (Perl AE, et al. N Engl J Med. 2019).
Aims
To determine whether prior TKI therapy affected response and survival in these two trials.
Methods
We retrospectively analyzed clinical outcomes in patients with R/R AML previously treated with TKIs midostaurin or sorafenib, before receiving 120- or 200-mg gilteritinib in the CHRYSALIS trial, or before receiving 120-mg gilteritinib in the ADMIRAL trial. Patients randomized to SC in the ADMIRAL trial were also assessed. Patients in the CHRYSALIS trial had received at least one line of prior AML therapy; patients in the ADMIRAL trial received only one line of prior AML therapy.
Results
Of the 145 FLT3-mutation–enriched patients who received 120- or 200-mg gilteritinib in the CHRYSALIS trial, 33 (23%; 120 mg, n=15; 200 mg, n=18) had received a prior TKI (all received sorafenib). Baseline characteristics among patients who received (n=33) or did not receive prior TKIs (n=112) were similar. Rates of composite complete remission (CRc) were similar in patients who received prior TKIs (42%; n=14/33) and in those who did not (43%; n=48/112). Among patients who received prior TKIs, rates of CRc were 53% (n=8/15) in the 120-mg dose group and 33% (n=6/18) in the 200-mg dose group ; rates of CRc in patients who did not receive prior TKIs were similar across both the 120- and 200-mg dose groups (44% [n=18/41] and 42% [n=30/71], respectively). Among patients treated with prior TKIs across the 120- or 200-mg dose groups (n=33), most (73%; n=24) had received ≥3 lines of any prior AML therapy.
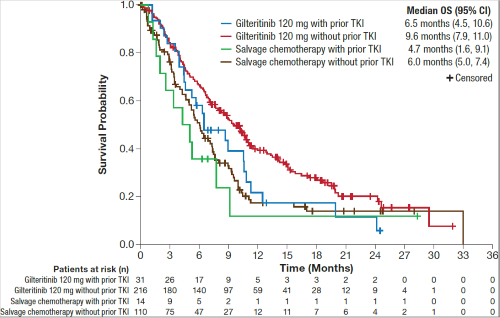
In the phase 3 ADMIRAL trial, 31 of 247 (13%) R/R FLT3-mutated AML patients in the gilteritinib arm and 14 of 124 (11%) patients in the SC arm had received prior TKIs. Demographic and baseline characteristics were well balanced between treatment arms and were also similar between prior TKI–treated (n=45) and non-treated patients (n=326). In the gilteritinib arm, CRc rates were comparable in patients who received (48%; n=15/31) and did not receive prior TKIs (55%; n=119/216); lower CRc rates were observed in the SC arm in both TKI–treated and non-treated groups (21% [n=3/14] and 22% [n=24/110], respectively). Median OS in patients treated with prior TKIs, albeit not statistically significant, remained high in patients treated with gilteritinib compared with those treated with SC (6.5 vs 4.7 months, respectively; HR=0.671 [95% CI: 0.328, 1.376]) (Figure). In patients who did not receive prior TKIs, median OS was 9.6 months in the gilteritinib arm and 6.0 months in the SC arm (HR=0.625 [95% CI: 0.474, 0.824]) (Figure).
Conclusion
Patients with R/R AML who received prior TKIs (midostaurin or sorafenib) were able to achieve remission with gilteritinib. High response rates with gilteritinib were observed in heavily pre-treated FLT3-mutation–enriched patients in the CHRYSALIS trial who received prior TKIs. Higher response rates with gilteritinib than with SC were observed in prior TKI–treated patients with FLT3 mutations in the ADMIRAL trial.
Keyword(s): Acute myeloid leukemia, FLT3, Tyrosine kinase inhibitor
Abstract: EP448
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Gilteritinib is a FLT3 inhibitor with demonstrated efficacy and safety in patients with FLT3-mutated relapsed or refractory (R/R) AML. The phase 1/2 CHRYSALIS trial demonstrated the safety and antileukemic activity of gilteritinib in a FLT3-mutation–enriched R/R AML population (Perl AE, et al. Lancet Oncol. 2017). The phase 3 ADMIRAL trial demonstrated the superiority of gilteritinib to salvage chemotherapy (SC) in FLT3-mutated patients based on longer median overall survival (OS) with gilteritinib (9.3 vs 5.6 months; hazard ratio [HR]=0.64 [95% CI: 0.49, 0.83]; P<0.001) (Perl AE, et al. N Engl J Med. 2019).
Aims
To determine whether prior TKI therapy affected response and survival in these two trials.
Methods
We retrospectively analyzed clinical outcomes in patients with R/R AML previously treated with TKIs midostaurin or sorafenib, before receiving 120- or 200-mg gilteritinib in the CHRYSALIS trial, or before receiving 120-mg gilteritinib in the ADMIRAL trial. Patients randomized to SC in the ADMIRAL trial were also assessed. Patients in the CHRYSALIS trial had received at least one line of prior AML therapy; patients in the ADMIRAL trial received only one line of prior AML therapy.
Results
Of the 145 FLT3-mutation–enriched patients who received 120- or 200-mg gilteritinib in the CHRYSALIS trial, 33 (23%; 120 mg, n=15; 200 mg, n=18) had received a prior TKI (all received sorafenib). Baseline characteristics among patients who received (n=33) or did not receive prior TKIs (n=112) were similar. Rates of composite complete remission (CRc) were similar in patients who received prior TKIs (42%; n=14/33) and in those who did not (43%; n=48/112). Among patients who received prior TKIs, rates of CRc were 53% (n=8/15) in the 120-mg dose group and 33% (n=6/18) in the 200-mg dose group ; rates of CRc in patients who did not receive prior TKIs were similar across both the 120- and 200-mg dose groups (44% [n=18/41] and 42% [n=30/71], respectively). Among patients treated with prior TKIs across the 120- or 200-mg dose groups (n=33), most (73%; n=24) had received ≥3 lines of any prior AML therapy.
In the phase 3 ADMIRAL trial, 31 of 247 (13%) R/R FLT3-mutated AML patients in the gilteritinib arm and 14 of 124 (11%) patients in the SC arm had received prior TKIs. Demographic and baseline characteristics were well balanced between treatment arms and were also similar between prior TKI–treated (n=45) and non-treated patients (n=326). In the gilteritinib arm, CRc rates were comparable in patients who received (48%; n=15/31) and did not receive prior TKIs (55%; n=119/216); lower CRc rates were observed in the SC arm in both TKI–treated and non-treated groups (21% [n=3/14] and 22% [n=24/110], respectively). Median OS in patients treated with prior TKIs, albeit not statistically significant, remained high in patients treated with gilteritinib compared with those treated with SC (6.5 vs 4.7 months, respectively; HR=0.671 [95% CI: 0.328, 1.376]) (Figure). In patients who did not receive prior TKIs, median OS was 9.6 months in the gilteritinib arm and 6.0 months in the SC arm (HR=0.625 [95% CI: 0.474, 0.824]) (Figure).

Conclusion
Patients with R/R AML who received prior TKIs (midostaurin or sorafenib) were able to achieve remission with gilteritinib. High response rates with gilteritinib were observed in heavily pre-treated FLT3-mutation–enriched patients in the CHRYSALIS trial who received prior TKIs. Higher response rates with gilteritinib than with SC were observed in prior TKI–treated patients with FLT3 mutations in the ADMIRAL trial.
Keyword(s): Acute myeloid leukemia, FLT3, Tyrosine kinase inhibitor


