
Contributions
Abstract: EP447
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Introduction. Midostaurin has been approved by EMA and FDA in combination with IC for FLT3-mutated AML based on improved overall survival noted in the RATIFY phase 3 trial (Stone et al, N Engl J Med 2017) The experience with midostaurin in day to day practice is limited.
Aims
Aim: The aim of this study is to analyze effectiveness and tolerability of midostaurin plus intensive chemotherapy (midos+IC) vs intensive chemotherapy alone (IC) as first lines in AML FLT3 positive patients and to identify risk factors.
Methods
Methods: We carried out the analysis on previously untreated AML FLT3 mutated patients included in PETHEMA AML epidemiological registry (NCT02607059). Selection criteria for the study were as follows: Age >18, diagnosis of AML FLT3 positive (ITD and TKD) under WHO criteria, treated with midos+IC or IC (IC defined as 7+3 induction and HiDAC or intermediate dose AraC consolidations) during the period 01/01/2010 to 01/03/2020, patients who died before day 7 of treatment were excluded. Response were assessed using the ELN-2010 criteria, toxicity by the CTCAE v4.0 scale, OS by Kaplan-Meier and early mortality as mortality within the first 8 weeks (M8wks).
Results
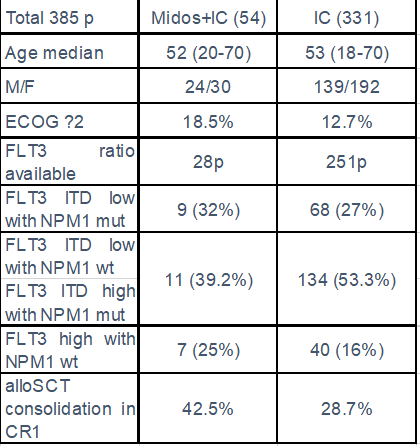
Results: A total of 385 of previously untreated AML FLT3 positive patients were analyzed (54 treated with Midos+IC and 331 with IC), median age 52yrs (20-70) and 53yrs (18-70), ECOG ≥2: 18.5% and 12.7% respectively. Baseline characteristics are summarized in table 1. Antifungal triazole prophylaxis was the standard in both cohorts. Early mortality M8wks was 3.7% and 6.6% and complete response rate (CR after 1 or 2 Inductions) 86% vs 66% (p 0.005) for midos+IC and IC respectively.
Overall survival is significantly longer in the mido+IC group than in the IC group (not reached vs 19 months, p=0.022), 24mOS 79.2% vs 54.2% (p0.026).
We observed a trend of benefit in all the following subgroups, a: FLT3 ITD low with NPM1 mut, b: FLT3 ITD low with NPM1 wt plus FLT3 ITD high with NPM1 mut; and c: FLT3 high with NPM1 wt. In our study we observe in midos+IC cohort a higher rate of patients consolidated in CR1 with alloSCT and a significantly longer OS for these patients (42.5% vs 28.7%) and (not reached vs 44.7 months).
Conclusion
Conclusions: The results of this study confirm in a real-world setting that midostaurin associated with IC improve the outcome of AML FLT3 positive patient compared versus IC alone.
Keyword(s): FLT3, Flt3 inhibitor, Leukemia
Abstract: EP447
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Introduction. Midostaurin has been approved by EMA and FDA in combination with IC for FLT3-mutated AML based on improved overall survival noted in the RATIFY phase 3 trial (Stone et al, N Engl J Med 2017) The experience with midostaurin in day to day practice is limited.
Aims
Aim: The aim of this study is to analyze effectiveness and tolerability of midostaurin plus intensive chemotherapy (midos+IC) vs intensive chemotherapy alone (IC) as first lines in AML FLT3 positive patients and to identify risk factors.
Methods
Methods: We carried out the analysis on previously untreated AML FLT3 mutated patients included in PETHEMA AML epidemiological registry (NCT02607059). Selection criteria for the study were as follows: Age >18, diagnosis of AML FLT3 positive (ITD and TKD) under WHO criteria, treated with midos+IC or IC (IC defined as 7+3 induction and HiDAC or intermediate dose AraC consolidations) during the period 01/01/2010 to 01/03/2020, patients who died before day 7 of treatment were excluded. Response were assessed using the ELN-2010 criteria, toxicity by the CTCAE v4.0 scale, OS by Kaplan-Meier and early mortality as mortality within the first 8 weeks (M8wks).
Results
Results: A total of 385 of previously untreated AML FLT3 positive patients were analyzed (54 treated with Midos+IC and 331 with IC), median age 52yrs (20-70) and 53yrs (18-70), ECOG ≥2: 18.5% and 12.7% respectively. Baseline characteristics are summarized in table 1. Antifungal triazole prophylaxis was the standard in both cohorts. Early mortality M8wks was 3.7% and 6.6% and complete response rate (CR after 1 or 2 Inductions) 86% vs 66% (p 0.005) for midos+IC and IC respectively.
Overall survival is significantly longer in the mido+IC group than in the IC group (not reached vs 19 months, p=0.022), 24mOS 79.2% vs 54.2% (p0.026).
We observed a trend of benefit in all the following subgroups, a: FLT3 ITD low with NPM1 mut, b: FLT3 ITD low with NPM1 wt plus FLT3 ITD high with NPM1 mut; and c: FLT3 high with NPM1 wt. In our study we observe in midos+IC cohort a higher rate of patients consolidated in CR1 with alloSCT and a significantly longer OS for these patients (42.5% vs 28.7%) and (not reached vs 44.7 months).

Conclusion
Conclusions: The results of this study confirm in a real-world setting that midostaurin associated with IC improve the outcome of AML FLT3 positive patient compared versus IC alone.
Keyword(s): FLT3, Flt3 inhibitor, Leukemia


