
Contributions
Abstract: EP446
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Maintenance therapy with oral azacitidine (AZA) significantly improved overall survival (OS) versus placebo in patients (pts) with acute myeloid leukemia (AML) who achieved remission after intensive induction chemotherapy in the phase 3 QUAZAR AML-001 study. Although there are no head-to-head trials comparing oral and injectable AZA, results from a recent indirect treatment comparison (ITC) favored oral AZA (Chen C, et al. ASH 2020); however, ITCs do not fully account for potentially effect-modifying variables between trials.
Aims
To compare the relative efficacy of maintenance therapy with oral versus injectable AZA in pts with AML in first remission after chemotherapy.
Methods
Anchored matching-adjusted indirect comparisons (MAICs) for OS were performed by matching individual pt data (IPD) from QUAZAR AML-001 (oral AZA vs placebo) to summary-level data from the comparator trials, HOVON97 (SC AZA vs control) and QoLESS (SC/IV AZA vs control). The QUAZAR AML-001 population was adjusted to comparators based on age, sex, AML disease stage, and cytogenetic risk; additionally, adjustment to HOVON97 included platelet count, response status at baseline, and ECOG performance status, and adjustment to QoLESS included minimal residual disease. After matching and adjustment, relative OS benefit was calculated within each trial as the difference in the Kaplan–Meier (KM) estimates of OS between the intervention and placebo/control arms, divided by the KM estimate of OS for placebo/control. The difference in restricted mean survival time (RMST) between intervention and placebo/control was estimated with a parametric survival model at 1, 2, 3, 4, and 5 years.
Results
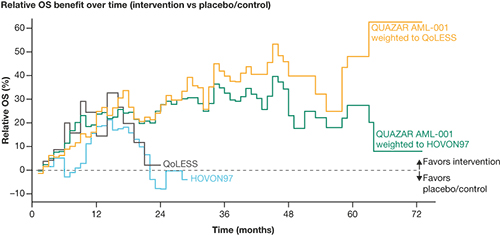
Of 472 pts in QUAZAR-AML-001, 402 and 328 were included in the analysis populations after matching to HOVON97 (N=116) and QoLESS (N=54), respectively; after adjustment, the effective sample size for the primary analysis population was reduced to 310 and 222 pts, respectively. Matching and adjustment led to small changes in estimates of oral AZA versus placebo/control compared with unweighted analyses. The weighted oral AZA population showed a sustained and numerically higher relative OS benefit through 60 months versus SC AZA and SC/IV AZA (Figure). The relative benefit of injectable AZA versus control observed in the initial 12 months appeared to fade, reaching close to zero around month 20. Difference in the area under the curve (∆AUC) between the weighted oral AZA populations and placebo/control showed improvement over time in point estimates of mean survival time. RMST from QUAZAR-AML-001 IPD weighted to HOVON97 showed a statistically meaningful improvement compared with placebo/control in mean survival time at 1, 2, 3, 4, and 5 years (∆AUC: 0.94, 2.43, 3.68, 4.68, and 5.48); a similar effect was observed with QUAZAR-AML-001 IPD weighted to QoLESS (∆AUC: 0.75, 2.41, 4.18, 5.83, and 7.32), whereas injectable AZA was not associated with significant benefits at any time-point in HOVON97 (∆AUC: 0.46, 0.95, 1.17, 1.18, and 1.08) or QoLESS (∆AUC: 1.12, 2.01, 2.11, 1.70, and 0.96). All scenario analyses conducted supported the results of the primary analyses.
Conclusion
Adjustment via MAIC resulted in consistent conclusions to the previously reported unadjusted analyses of oral versus injectable AZA. Weighted analysis of oral versus injectable AZA continued to show early and sustained relative OS benefits with oral AZA. Additionally, weighted estimates of oral AZA showed statistically meaningful improvement in mean survival time versus placebo/control, and numerical superiority to injectable AZA.
Keyword(s): Acute myeloid leukemia, Azacitidine, Maintenance, Outcome
Abstract: EP446
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Maintenance therapy with oral azacitidine (AZA) significantly improved overall survival (OS) versus placebo in patients (pts) with acute myeloid leukemia (AML) who achieved remission after intensive induction chemotherapy in the phase 3 QUAZAR AML-001 study. Although there are no head-to-head trials comparing oral and injectable AZA, results from a recent indirect treatment comparison (ITC) favored oral AZA (Chen C, et al. ASH 2020); however, ITCs do not fully account for potentially effect-modifying variables between trials.
Aims
To compare the relative efficacy of maintenance therapy with oral versus injectable AZA in pts with AML in first remission after chemotherapy.
Methods
Anchored matching-adjusted indirect comparisons (MAICs) for OS were performed by matching individual pt data (IPD) from QUAZAR AML-001 (oral AZA vs placebo) to summary-level data from the comparator trials, HOVON97 (SC AZA vs control) and QoLESS (SC/IV AZA vs control). The QUAZAR AML-001 population was adjusted to comparators based on age, sex, AML disease stage, and cytogenetic risk; additionally, adjustment to HOVON97 included platelet count, response status at baseline, and ECOG performance status, and adjustment to QoLESS included minimal residual disease. After matching and adjustment, relative OS benefit was calculated within each trial as the difference in the Kaplan–Meier (KM) estimates of OS between the intervention and placebo/control arms, divided by the KM estimate of OS for placebo/control. The difference in restricted mean survival time (RMST) between intervention and placebo/control was estimated with a parametric survival model at 1, 2, 3, 4, and 5 years.
Results
Of 472 pts in QUAZAR-AML-001, 402 and 328 were included in the analysis populations after matching to HOVON97 (N=116) and QoLESS (N=54), respectively; after adjustment, the effective sample size for the primary analysis population was reduced to 310 and 222 pts, respectively. Matching and adjustment led to small changes in estimates of oral AZA versus placebo/control compared with unweighted analyses. The weighted oral AZA population showed a sustained and numerically higher relative OS benefit through 60 months versus SC AZA and SC/IV AZA (Figure). The relative benefit of injectable AZA versus control observed in the initial 12 months appeared to fade, reaching close to zero around month 20. Difference in the area under the curve (∆AUC) between the weighted oral AZA populations and placebo/control showed improvement over time in point estimates of mean survival time. RMST from QUAZAR-AML-001 IPD weighted to HOVON97 showed a statistically meaningful improvement compared with placebo/control in mean survival time at 1, 2, 3, 4, and 5 years (∆AUC: 0.94, 2.43, 3.68, 4.68, and 5.48); a similar effect was observed with QUAZAR-AML-001 IPD weighted to QoLESS (∆AUC: 0.75, 2.41, 4.18, 5.83, and 7.32), whereas injectable AZA was not associated with significant benefits at any time-point in HOVON97 (∆AUC: 0.46, 0.95, 1.17, 1.18, and 1.08) or QoLESS (∆AUC: 1.12, 2.01, 2.11, 1.70, and 0.96). All scenario analyses conducted supported the results of the primary analyses.

Conclusion
Adjustment via MAIC resulted in consistent conclusions to the previously reported unadjusted analyses of oral versus injectable AZA. Weighted analysis of oral versus injectable AZA continued to show early and sustained relative OS benefits with oral AZA. Additionally, weighted estimates of oral AZA showed statistically meaningful improvement in mean survival time versus placebo/control, and numerical superiority to injectable AZA.
Keyword(s): Acute myeloid leukemia, Azacitidine, Maintenance, Outcome


