
Contributions
Abstract: EP445
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Most older patients (pts) who attain AML remission with intensive chemotherapy (IC) eventually relapse. Oral-AZA is a hypomethylating agent (HMA) that allows for extended dosing schedules to sustain drug activity. In the phase 3 QUAZAR AML-001 trial, Oral-AZA significantly prolonged overall and relapse-free survival (OS/RFS) vs placebo (PBO) in older pts with AML in first remission after IC. Neutropenia (N), thrombocytopenia (T), and anemia (A) were the most common hematologic adverse events (AEs) in both treatment (Tx) arms, occurring more often with Oral-AZA vs PBO. Cytopenias are a known side effect of HMAs, especially during early Tx, but may also portend AML relapse. Thus, it can be challenging to discern whether cytopenias during Oral-AZA Tx are related to Tx or to disease progression.
Aims
Assess the rate of cytopenias over time, via AE reporting and laboratory parameters, for pts receiving Oral-AZA in the QUAZAR AML-001 study, and determine whether cytopenias presaged impending AML relapse.
Methods
Pts were aged ≥55 yrs, had intermediate- or poor-risk cytogenetics and ECOG PS score ≤3, and were not candidates for transplant. Within 4 months (mo) of achieving first complete remission (CR) or CR with incomplete blood count recovery (CRi) after induction ± consolidation, pts were randomized 1:1 to Oral-AZA 300 mg or PBO QD for 14d/28d Tx cycles. Pts who received ≥1 study drug dose were monitored for AEs from first dose until 28d after last dose.
In these post hoc analyses, we report hematology parameters (ANC, platelets, hemoglobin [Hgb]) for pts who relapsed on-study at baseline (BL), at 1–2 mo (≤60 to >30d) before relapse, and during the 1 mo (≤30d) before relapse, inclusive of relapse date. For context, we also assessed these parameters at BL and at cycles 3, 6, and 9 for pts who did not relapse on-study.
Results
469 pts (Oral-AZA 236, PBO 233) were followed for safety. Median age was 68 yrs (range 55–86). Pts received a median of 12 (range 1-80) Oral-AZA Tx cycles and 6 (1-73) PBO cycles.
Rates of any grade N, T, and A in the Oral-AZA arm were 44%, 33%, and 20%, respectively. At cycles 1–2, 3–4, and 5–6, rates of N in the Oral-AZA arm were 22%, 20%, and 18%, respectively; of T were 16%, 9%, and 6%; and of A were 6%, 4%, and 4%. Oral-AZA Tx was interrupted or dose reduced, respectively, in 45% and 12% of 105 pts who experienced N; in 25% and 5% of 79 pts with T; and in 6% and 2% of 48 pts with A. Only 1 pt (1%) discontinued Oral-AZA due to N, T, or A (T). 23% of pts in the Oral-AZA arm (PBO 22%) received RBC transfusions on-study and 19% (PBO 22%) received platelet transfusions. G-CSF use was infrequent: 8 pts (3.4%) in the Oral-AZA arm and 7 pts (3%) in the PBO arm.
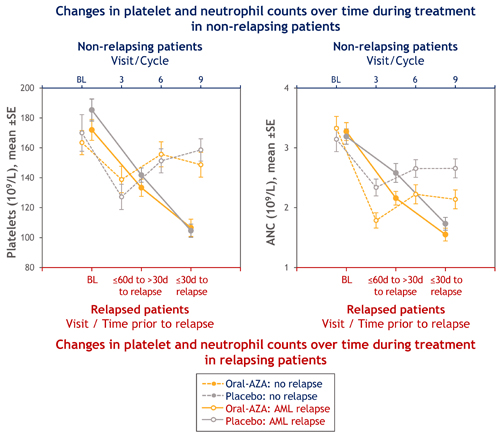
154 Oral-AZA pts (65%) and 179 PBO pts (77%) relapsed on-study; median RFS was 10.2 vs 4.8 mo, respectively (P<0.001). Pts who relapsed showed steep decreases in ANC and platelets (Figure) but not Hgb, in the 2 mo before AML relapse. Pts who did not relapse showed initial decreases in platelet counts and ANC, but signs of recovery were evident after cycle 3 (Figure).
Conclusion
Hematologic AEs with Oral-AZA, particularly N, occurred most often during early Tx. N, T, and A were mainly managed with temporary Tx interruptions. Infrequent G-CSF use and rare Tx discontinuation indicate cytopenias were manageable with dosing modifications. Non-relapsing pts showed early decreases in platelets and ANC, with recovery after cycle 3. Relapsing pts showed steep declines in platelets and ANC with clear differences from non-relapsing pts within 30d of relapse.
Keyword(s): AML, Azacitidine, Oral, Safety
Abstract: EP445
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Most older patients (pts) who attain AML remission with intensive chemotherapy (IC) eventually relapse. Oral-AZA is a hypomethylating agent (HMA) that allows for extended dosing schedules to sustain drug activity. In the phase 3 QUAZAR AML-001 trial, Oral-AZA significantly prolonged overall and relapse-free survival (OS/RFS) vs placebo (PBO) in older pts with AML in first remission after IC. Neutropenia (N), thrombocytopenia (T), and anemia (A) were the most common hematologic adverse events (AEs) in both treatment (Tx) arms, occurring more often with Oral-AZA vs PBO. Cytopenias are a known side effect of HMAs, especially during early Tx, but may also portend AML relapse. Thus, it can be challenging to discern whether cytopenias during Oral-AZA Tx are related to Tx or to disease progression.
Aims
Assess the rate of cytopenias over time, via AE reporting and laboratory parameters, for pts receiving Oral-AZA in the QUAZAR AML-001 study, and determine whether cytopenias presaged impending AML relapse.
Methods
Pts were aged ≥55 yrs, had intermediate- or poor-risk cytogenetics and ECOG PS score ≤3, and were not candidates for transplant. Within 4 months (mo) of achieving first complete remission (CR) or CR with incomplete blood count recovery (CRi) after induction ± consolidation, pts were randomized 1:1 to Oral-AZA 300 mg or PBO QD for 14d/28d Tx cycles. Pts who received ≥1 study drug dose were monitored for AEs from first dose until 28d after last dose.
In these post hoc analyses, we report hematology parameters (ANC, platelets, hemoglobin [Hgb]) for pts who relapsed on-study at baseline (BL), at 1–2 mo (≤60 to >30d) before relapse, and during the 1 mo (≤30d) before relapse, inclusive of relapse date. For context, we also assessed these parameters at BL and at cycles 3, 6, and 9 for pts who did not relapse on-study.
Results
469 pts (Oral-AZA 236, PBO 233) were followed for safety. Median age was 68 yrs (range 55–86). Pts received a median of 12 (range 1-80) Oral-AZA Tx cycles and 6 (1-73) PBO cycles.
Rates of any grade N, T, and A in the Oral-AZA arm were 44%, 33%, and 20%, respectively. At cycles 1–2, 3–4, and 5–6, rates of N in the Oral-AZA arm were 22%, 20%, and 18%, respectively; of T were 16%, 9%, and 6%; and of A were 6%, 4%, and 4%. Oral-AZA Tx was interrupted or dose reduced, respectively, in 45% and 12% of 105 pts who experienced N; in 25% and 5% of 79 pts with T; and in 6% and 2% of 48 pts with A. Only 1 pt (1%) discontinued Oral-AZA due to N, T, or A (T). 23% of pts in the Oral-AZA arm (PBO 22%) received RBC transfusions on-study and 19% (PBO 22%) received platelet transfusions. G-CSF use was infrequent: 8 pts (3.4%) in the Oral-AZA arm and 7 pts (3%) in the PBO arm.
154 Oral-AZA pts (65%) and 179 PBO pts (77%) relapsed on-study; median RFS was 10.2 vs 4.8 mo, respectively (P<0.001). Pts who relapsed showed steep decreases in ANC and platelets (Figure) but not Hgb, in the 2 mo before AML relapse. Pts who did not relapse showed initial decreases in platelet counts and ANC, but signs of recovery were evident after cycle 3 (Figure).

Conclusion
Hematologic AEs with Oral-AZA, particularly N, occurred most often during early Tx. N, T, and A were mainly managed with temporary Tx interruptions. Infrequent G-CSF use and rare Tx discontinuation indicate cytopenias were manageable with dosing modifications. Non-relapsing pts showed early decreases in platelets and ANC, with recovery after cycle 3. Relapsing pts showed steep declines in platelets and ANC with clear differences from non-relapsing pts within 30d of relapse.
Keyword(s): AML, Azacitidine, Oral, Safety


