
Contributions
Abstract: EP442
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
CPX-351 (EU: Vyxeos® Liposomal; US: Vyxeos®), a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a synergistic 1:5 molar drug ratio, is approved by the EMA and US FDA for the treatment of adults with newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes. Preclinical data suggest CPX-351 may exert synergistic activity when combined with agents such as the BCL-2 inhibitor venetoclax or FLT3 inhibitor midostaurin.
Aims
The objective of the V-FAST study is to evaluate the safety and establish the recommended phase 2 dose (RP2D) of CPX-351 combined with other agents for the treatment of adults with previously untreated AML.
Methods
V-FAST (Vyxeos – First Phase Assessment With Targeted Agents) is an open-label, multicenter, phase 1b master trial (NCT04075747) to evaluate CPX-351 in combination with targeted agents in patients aged 18 to 75 years with untreated AML who are fit for intensive chemotherapy. The study includes a dose-exploration phase (3+3 design) and subsequent expansion phase. Patients received CPX-351 (dose level 1 for first induction [DL1]: 100 units/m2 on Days 1, 3, and 5) plus venetoclax (Arm A; DL1: 400 mg on Days 1 to 14), midostaurin (Arm B; DL1: 50 mg BID on Days 8 to 21), or the IDH2 inhibitor enasidenib (Arm C; DL1: 100 mg on Days 8 to 28) based on mutation testing.
Results
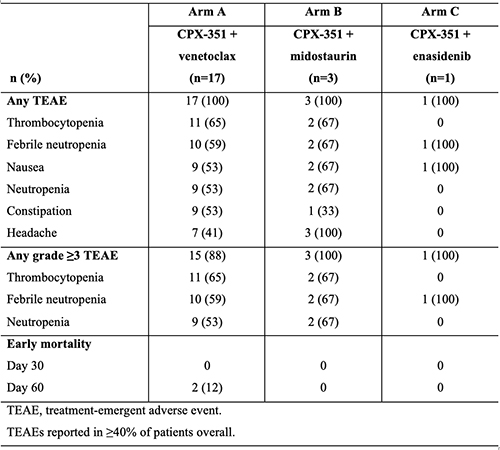
Among 21 patients with available data enrolled by 06 November 2020 (24 pts enrolled total; data cut-off: 19 January 2021), the median age was 54 years (range: 35, 69). In Arm A (n=17), 11 (65%) patients had de novo AML, 5 (29%) had an antecedent hematologic disorder (2 [12%] had myelofibrosis), and 2 (12%) had t-AML; 12 (71%) had adverse-risk AML; and 6 (35%) had mutated TP53. In Arms B (n=3) and C (n=1), all patients had intermediate-risk de novo AML. DL1 was the RP2D in Arms A and B; the RP2D in Arm C is still under investigation. In Arm A, 1 of 6 patients in the dose-exploration phase had 2 dose-limiting toxicities (DLTs) of grade 4 neutropenia and thrombocytopenia that extended beyond 49 days; no DLTs have occurred for Arms B and C. The combinations exhibited manageable safety profiles (Table). Of patients with available response data, complete remission (CR) or CR with incomplete platelet or neutrophil recovery was achieved by 6 of 14 (43%) patients in Arm A, including 4 (29%) with CR. All patientts in Arms B and C achieved CR.
Conclusion
These preliminary results suggest CPX-351 can be combined with venetoclax or midostaurin with manageable toxicities in newly diagnosed AML patients, with DL1 determined to be the RP2D. The study is ongoing and actively enrolling patients; updated results will be presented at the meeting.
Keyword(s): Acute myeloid leukemia, Chemotherapy, Phase I, Targeted therapy
Abstract: EP442
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
CPX-351 (EU: Vyxeos® Liposomal; US: Vyxeos®), a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a synergistic 1:5 molar drug ratio, is approved by the EMA and US FDA for the treatment of adults with newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes. Preclinical data suggest CPX-351 may exert synergistic activity when combined with agents such as the BCL-2 inhibitor venetoclax or FLT3 inhibitor midostaurin.
Aims
The objective of the V-FAST study is to evaluate the safety and establish the recommended phase 2 dose (RP2D) of CPX-351 combined with other agents for the treatment of adults with previously untreated AML.
Methods
V-FAST (Vyxeos – First Phase Assessment With Targeted Agents) is an open-label, multicenter, phase 1b master trial (NCT04075747) to evaluate CPX-351 in combination with targeted agents in patients aged 18 to 75 years with untreated AML who are fit for intensive chemotherapy. The study includes a dose-exploration phase (3+3 design) and subsequent expansion phase. Patients received CPX-351 (dose level 1 for first induction [DL1]: 100 units/m2 on Days 1, 3, and 5) plus venetoclax (Arm A; DL1: 400 mg on Days 1 to 14), midostaurin (Arm B; DL1: 50 mg BID on Days 8 to 21), or the IDH2 inhibitor enasidenib (Arm C; DL1: 100 mg on Days 8 to 28) based on mutation testing.
Results
Among 21 patients with available data enrolled by 06 November 2020 (24 pts enrolled total; data cut-off: 19 January 2021), the median age was 54 years (range: 35, 69). In Arm A (n=17), 11 (65%) patients had de novo AML, 5 (29%) had an antecedent hematologic disorder (2 [12%] had myelofibrosis), and 2 (12%) had t-AML; 12 (71%) had adverse-risk AML; and 6 (35%) had mutated TP53. In Arms B (n=3) and C (n=1), all patients had intermediate-risk de novo AML. DL1 was the RP2D in Arms A and B; the RP2D in Arm C is still under investigation. In Arm A, 1 of 6 patients in the dose-exploration phase had 2 dose-limiting toxicities (DLTs) of grade 4 neutropenia and thrombocytopenia that extended beyond 49 days; no DLTs have occurred for Arms B and C. The combinations exhibited manageable safety profiles (Table). Of patients with available response data, complete remission (CR) or CR with incomplete platelet or neutrophil recovery was achieved by 6 of 14 (43%) patients in Arm A, including 4 (29%) with CR. All patientts in Arms B and C achieved CR.

Conclusion
These preliminary results suggest CPX-351 can be combined with venetoclax or midostaurin with manageable toxicities in newly diagnosed AML patients, with DL1 determined to be the RP2D. The study is ongoing and actively enrolling patients; updated results will be presented at the meeting.
Keyword(s): Acute myeloid leukemia, Chemotherapy, Phase I, Targeted therapy


