
Contributions
Abstract: EP437
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Gilteritinib, an oral FMS-like tyrosine kinase 3 (FLT3) inhibitor, demonstrated antileukemic responses in patients (pts) with FLT3-mutated (FLT3mut) relapsed/refractory acute myeloid leukemia (AML).
Aims
We report updated final data from a phase 1 study of once-daily oral gilteritinib + intravenous chemotherapy in pts with newly diagnosed (ND) AML.
Methods
As detailed previously in Pratz et al. Blood 2020;136(supp1):16-17, this 4-part, open-label, phase 1 study (NCT02236013) assessed the safety/tolerability and antileukemic effects of gilteritinib plus 7+3 induction and ≤3 cycles of high-dose cytarabine consolidation, and as single-agent maintenance treatment (tx) in adults with ND AML. During induction, cohorts varied in their gilteritinib dose (40–200 mg during dose escalation and 120 mg in cohort expansion), gilteritinib administration schedule (days 4–17 or 8–21), and anthracycline choice (idarubicin or daunorubicin). Eligible patients could receive a second 7+3+gilteritinib induction cycle. During consolidation, gilteritinib was administered at induction dose on days 1–14 or 1–56 of each cycle (cohort dependent). During maintenance, gilteritinib was given daily (28-day cycles) for up to 26 cycles. Pts with a composite complete remission (CRc) or partial remission could undergo hematopoietic stem cell transplant (HSCT) and resume maintenance gilteritinib tx post HSCT. Safety and tolerability were the primary endpoints.
Results
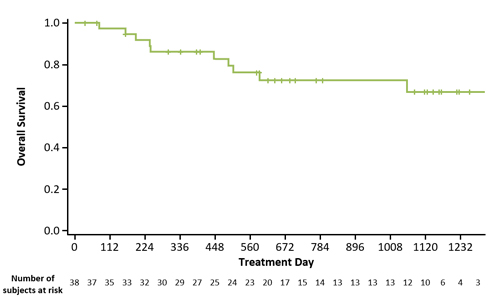
As of 23 June 2020, 80 pts were allocated to tx (safety analysis set, n=79); median age was 59.0 y (range, 23–77) and 44 were FLT3mut. Median follow-up was 35.8 mo. HSCT was performed in 30.4% (24/79) of pts. Dose-limiting toxicities (DLTs) occurred in 15/79 (19.0%) pts. DLTs in the 200 mg/d cohort were prolonged neutropenia (n=1) and neutropenic enterocolitis (n=1). The maximum tolerated dose was 120 mg/d. Serious tx-related adverse events (AEs) and AEs leading to discontinuation of gilteritinib occurred in 29.1% (n=23) and 22.8% (n=18) of pts, respectively. Grade ≥3 nonhematologic AEs (≥10% of pts) were increased alanine aminotransferase (13.9%), pneumonia (13.9%), sepsis (11.4%), and bacteremia (11.4%). A 120 mg/d dose of gilteritinib was received by 38 (86.4%) FLT3mut pts. In Pratz et al. Blood 2020, we reported clinical responses at end-of-induction but now report response at end-of-treatment, consistent with previous disclosures. Among FLT3mut pts who received gilteritinib 120 mg/d, the CRc rate was 89.5% (complete remission [CR], 71.1%; CR with incomplete hematological recovery, 18.4%) and median overall survival has not been reached (Figure). Anthracycline choice had no significant impact on CRc rate or toxicity. Estimated 1- and 2-year survival probabilities were 85.9% and 72.3% respectively. The 60-day mortality rate was 0%. Median (95% CI) disease-free survival was 13.3 (4.9–18.7) mo. In pts who received gilteritinib 120 mg/d and achieved CRc, mutational clearance of FLT3 internal tandem duplication (ITD) (summed FLT3 ITD:WT signal ratio of ≤10-4) after induction or consolidation was 84.6% (11/13).
Conclusion
Gilteritinib + induction and consolidation chemotherapy is well-tolerated in pts with ND FLT3mut AML and often leads to CR, clearance of FLT3 ITD, and long-term survival. Based on these data, randomized trials of gilteritinib vs midostaurin as part of intensive frontline therapy of FLT3mut AML have been initiated.
Keyword(s): Acute myeloid leukemia, Flt3 inhibitor
Abstract: EP437
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Gilteritinib, an oral FMS-like tyrosine kinase 3 (FLT3) inhibitor, demonstrated antileukemic responses in patients (pts) with FLT3-mutated (FLT3mut) relapsed/refractory acute myeloid leukemia (AML).
Aims
We report updated final data from a phase 1 study of once-daily oral gilteritinib + intravenous chemotherapy in pts with newly diagnosed (ND) AML.
Methods
As detailed previously in Pratz et al. Blood 2020;136(supp1):16-17, this 4-part, open-label, phase 1 study (NCT02236013) assessed the safety/tolerability and antileukemic effects of gilteritinib plus 7+3 induction and ≤3 cycles of high-dose cytarabine consolidation, and as single-agent maintenance treatment (tx) in adults with ND AML. During induction, cohorts varied in their gilteritinib dose (40–200 mg during dose escalation and 120 mg in cohort expansion), gilteritinib administration schedule (days 4–17 or 8–21), and anthracycline choice (idarubicin or daunorubicin). Eligible patients could receive a second 7+3+gilteritinib induction cycle. During consolidation, gilteritinib was administered at induction dose on days 1–14 or 1–56 of each cycle (cohort dependent). During maintenance, gilteritinib was given daily (28-day cycles) for up to 26 cycles. Pts with a composite complete remission (CRc) or partial remission could undergo hematopoietic stem cell transplant (HSCT) and resume maintenance gilteritinib tx post HSCT. Safety and tolerability were the primary endpoints.
Results
As of 23 June 2020, 80 pts were allocated to tx (safety analysis set, n=79); median age was 59.0 y (range, 23–77) and 44 were FLT3mut. Median follow-up was 35.8 mo. HSCT was performed in 30.4% (24/79) of pts. Dose-limiting toxicities (DLTs) occurred in 15/79 (19.0%) pts. DLTs in the 200 mg/d cohort were prolonged neutropenia (n=1) and neutropenic enterocolitis (n=1). The maximum tolerated dose was 120 mg/d. Serious tx-related adverse events (AEs) and AEs leading to discontinuation of gilteritinib occurred in 29.1% (n=23) and 22.8% (n=18) of pts, respectively. Grade ≥3 nonhematologic AEs (≥10% of pts) were increased alanine aminotransferase (13.9%), pneumonia (13.9%), sepsis (11.4%), and bacteremia (11.4%). A 120 mg/d dose of gilteritinib was received by 38 (86.4%) FLT3mut pts. In Pratz et al. Blood 2020, we reported clinical responses at end-of-induction but now report response at end-of-treatment, consistent with previous disclosures. Among FLT3mut pts who received gilteritinib 120 mg/d, the CRc rate was 89.5% (complete remission [CR], 71.1%; CR with incomplete hematological recovery, 18.4%) and median overall survival has not been reached (Figure). Anthracycline choice had no significant impact on CRc rate or toxicity. Estimated 1- and 2-year survival probabilities were 85.9% and 72.3% respectively. The 60-day mortality rate was 0%. Median (95% CI) disease-free survival was 13.3 (4.9–18.7) mo. In pts who received gilteritinib 120 mg/d and achieved CRc, mutational clearance of FLT3 internal tandem duplication (ITD) (summed FLT3 ITD:WT signal ratio of ≤10-4) after induction or consolidation was 84.6% (11/13).

Conclusion
Gilteritinib + induction and consolidation chemotherapy is well-tolerated in pts with ND FLT3mut AML and often leads to CR, clearance of FLT3 ITD, and long-term survival. Based on these data, randomized trials of gilteritinib vs midostaurin as part of intensive frontline therapy of FLT3mut AML have been initiated.
Keyword(s): Acute myeloid leukemia, Flt3 inhibitor


