
Contributions
Abstract: EP436
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Mutations in the IDH1 and IDH2 genes belong to the most frequent recurrent genetic alterations in AML. Whereas IDH mutations in total do not have a favorable nor adverse prognostic impact, certain subtypes may be associated with a more favorable or adverse clinical course. The majority of available data on prevalence and prognostic impact was obtained in selected patient groups, who were most commonly treated intensively as part of clinical trial protocols.
Aims
We set up a study to prospectively screen for IDH mutations and collect prognostic information on IDH in a large real-world cohort of patients (pts) enrolled in the AML registry of the SAL study group.
Methods
Between April 2016 and March 2020, all pts from centers participating in the SAL registry were screened for IDH mutations by a sensitive amplicon-based NGS approach. Both newly diagnosed and relapsed/refractory (r/r) pts receiving either intensive or non-intensive treatments were screened. Pts did not receive IDH inhibitors. The study was approved by the responsible Ethics Committee as part of the SAL AML registry (NCT03188874).
Results
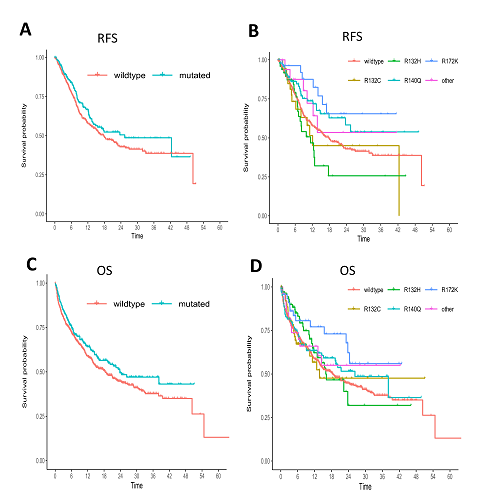
2690 pts were screened, of which 2457 were obtained at initial diagnosis and 233 in r/r AML. The prevalence of IDH1 and IDH2 mutations was 9.7% and 13.3%, respectively, which was identical at initial diagnosis and in r/r disease. Mutations R132C and R132H were the most common mutations in IDH1, whereas in IDH2, R140Q and R172K were most frequently found.
In newly diagnosed AML, the CR rate in IDH-mut versus (vs) IDH-wt was 68% vs 67% (p=0.919). The median relapse-free survival (RFS) in mut vs wt pts was 25.4 vs 17.8 months (m) (p=0.106). Subgroup analyses revealed a superior survival of IDH2-R172K mut pts with a 1-year RFS of 88% vs 58% in IDH-wt pts (p=0.037). A trend for superior overall survival (OS) was observed in IDH-mut pts with a median OS of 24 vs 18 m (p=0.066). IDH2-R172K pts had a significantly better prognosis than IDH-wt pts with a 1-year OS of 77% vs 59% (p=0.037). The described patterns were similar if pts eligible for intensive treatment were analysed separately.
In 137 pts with de novo AML ineligible for intensive treatment, median RFS in pts achieving a CR was longer in IDH-mut vs IDH-wt (8.5 vs 6.3 m; p=0.041); however, median OS in all unfit pts did not differ significantly between IDH-mut and IDH-wt (4.9 vs 4.3 m; p=0.326).
In 105 evaluable r/r AML, the CR rate in IDH-mut vs IDH-wt was 42% vs 59% (p=0.132), 1-year RFS 67% vs 40% (p=0.309) and 1-year OS identical with 67%.
Figure: RFS and OS depending on IDH mutational status in newly diagnosed AML (IDH1-R132C, IDH1-R132H, IDH2-R149Q, IDH2-R172K). A, RFS in IDH-mut vs IDH-wt; B, RFS in IDH-mut subgroups vs IDH-wt; C, OS in IDH-mut vs IDH-wt; D, OS in IDH-mut subgroups vs IDH-wt.
Conclusion
In this prospectively analyzed cohort of AML pts from a real-world setting, we detected IDH mutations in 23% of cases, irrespective of the disease status. Whereas IDH mutations were not associated with differences in RFS and OS in the total cohort, significantly prolonged survival outcomes could be identified for the subgroup of newly diagnosed pts with IDH2-R172K. Our results confirm the favorable prognosis of the IDH2-R172K subgroup. Furthermore, the results from this real-world cohort including r/r AMLs and pts ineligible for intensive treatment match the results of previously published newly diagnosed and fit patient groups from clinical trials.[1]
[1] Middeke JM, Metzeler KH, Röllig C et al. Clinical characteristics and outcome in IDH1/2 mutant AML pts. Oncol Res Treat 2019;42(suppl 4): abstract V1022
Keyword(s): AML, Mutation status, Prognosis
Abstract: EP436
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Mutations in the IDH1 and IDH2 genes belong to the most frequent recurrent genetic alterations in AML. Whereas IDH mutations in total do not have a favorable nor adverse prognostic impact, certain subtypes may be associated with a more favorable or adverse clinical course. The majority of available data on prevalence and prognostic impact was obtained in selected patient groups, who were most commonly treated intensively as part of clinical trial protocols.
Aims
We set up a study to prospectively screen for IDH mutations and collect prognostic information on IDH in a large real-world cohort of patients (pts) enrolled in the AML registry of the SAL study group.
Methods
Between April 2016 and March 2020, all pts from centers participating in the SAL registry were screened for IDH mutations by a sensitive amplicon-based NGS approach. Both newly diagnosed and relapsed/refractory (r/r) pts receiving either intensive or non-intensive treatments were screened. Pts did not receive IDH inhibitors. The study was approved by the responsible Ethics Committee as part of the SAL AML registry (NCT03188874).
Results
2690 pts were screened, of which 2457 were obtained at initial diagnosis and 233 in r/r AML. The prevalence of IDH1 and IDH2 mutations was 9.7% and 13.3%, respectively, which was identical at initial diagnosis and in r/r disease. Mutations R132C and R132H were the most common mutations in IDH1, whereas in IDH2, R140Q and R172K were most frequently found.
In newly diagnosed AML, the CR rate in IDH-mut versus (vs) IDH-wt was 68% vs 67% (p=0.919). The median relapse-free survival (RFS) in mut vs wt pts was 25.4 vs 17.8 months (m) (p=0.106). Subgroup analyses revealed a superior survival of IDH2-R172K mut pts with a 1-year RFS of 88% vs 58% in IDH-wt pts (p=0.037). A trend for superior overall survival (OS) was observed in IDH-mut pts with a median OS of 24 vs 18 m (p=0.066). IDH2-R172K pts had a significantly better prognosis than IDH-wt pts with a 1-year OS of 77% vs 59% (p=0.037). The described patterns were similar if pts eligible for intensive treatment were analysed separately.
In 137 pts with de novo AML ineligible for intensive treatment, median RFS in pts achieving a CR was longer in IDH-mut vs IDH-wt (8.5 vs 6.3 m; p=0.041); however, median OS in all unfit pts did not differ significantly between IDH-mut and IDH-wt (4.9 vs 4.3 m; p=0.326).
In 105 evaluable r/r AML, the CR rate in IDH-mut vs IDH-wt was 42% vs 59% (p=0.132), 1-year RFS 67% vs 40% (p=0.309) and 1-year OS identical with 67%.
Figure: RFS and OS depending on IDH mutational status in newly diagnosed AML (IDH1-R132C, IDH1-R132H, IDH2-R149Q, IDH2-R172K). A, RFS in IDH-mut vs IDH-wt; B, RFS in IDH-mut subgroups vs IDH-wt; C, OS in IDH-mut vs IDH-wt; D, OS in IDH-mut subgroups vs IDH-wt.

Conclusion
In this prospectively analyzed cohort of AML pts from a real-world setting, we detected IDH mutations in 23% of cases, irrespective of the disease status. Whereas IDH mutations were not associated with differences in RFS and OS in the total cohort, significantly prolonged survival outcomes could be identified for the subgroup of newly diagnosed pts with IDH2-R172K. Our results confirm the favorable prognosis of the IDH2-R172K subgroup. Furthermore, the results from this real-world cohort including r/r AMLs and pts ineligible for intensive treatment match the results of previously published newly diagnosed and fit patient groups from clinical trials.[1]
[1] Middeke JM, Metzeler KH, Röllig C et al. Clinical characteristics and outcome in IDH1/2 mutant AML pts. Oncol Res Treat 2019;42(suppl 4): abstract V1022
Keyword(s): AML, Mutation status, Prognosis


