
Contributions
Abstract: EP435
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
In acute myeloid leukemia (AML), mutations in the genes DNMT3A, TET2, and ASXL1 (DTA) are frequent and appear early in leukemogenesis. In older, otherwise healthy individuals DTA mutations may be present as clonal hematopoiesis of indeterminate potential (CHIP). While TET2 mutations do not have a common hotspot, mutations in DNMT3A and ASXL1 frequently occur at specific positions (DNMT3A in R882 and ASXL1 as G646fs*12, referred to as “canonical”). The persistence of gene mutations prior to allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation therapy may identify AML patients (pts) at high-risk of relapse. Until today, no study genuinely evaluated the applicability of DTA mutations - including canonical vs. non-canonical mutations - as residual disease markers in AML pts receiving HSCT consolidation.
Aims
To evaluate DTA mutations as residual AML disease markers in pts prior to allogeneic HSCT.
Methods
We analyzed 68 AML pts harboring at least one DTA mutation (DNMT3A, n=41; TET2, n=18; ASXL1, n=15) at diagnosis, who received HSCT (median age 64, range 35-75 years) in complete remission (CR; n=50) or CR with incomplete recovery (CRi, n=18). Conditioning regimens were non-myeloablative (n=57), reduced intensity (n=7) or myeloablative (n=4). At diagnosis, the mutational status of 54 genes was evaluated using next generation sequencing at diagnosis. Applying highly sensitive mutation-specific digital droplet PCR (ddPCR) assays at the timepoint of HSCT, we tested for the persistence of DTA mutations. Median follow up was 5.0 years.
Results
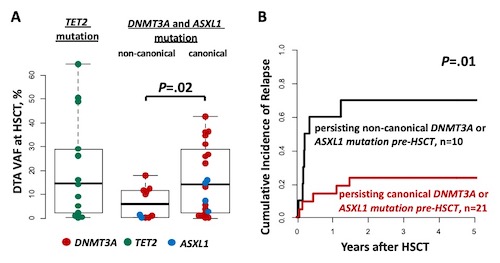
At diagnosis, 41 pts had a DNMT3A mutation (62%; canonical: 61%; non-canonical: 39%) 18 had a TET2 mutation (33%) and 15 had an ASXL1 mutation (22%; canonical: 80%; non-canonical: 20%). Most frequently co-mutated genes were NPM1 (43%), RUNX1 (19%), IDH2 (16%), and BCOR, IDH1, and FLT3-ITD (each with 15%). At HSCT, DTA mutations persisted in the majority of pts (overall 84%; DNMT3A 86%, TET2 93%, and ASXL1 60%). The rate of sustained detection did not differ significantly between canonical and non-canonical mutations (DNMT3A: 89% vs 80%, P=1 and ASXL1: 57% vs 67%, P=1). Of the analyzed non-canonical mutations in DNMT3A 10% were frameshift, 60% were missense, and 30% were nonsense, while in ASXL1 all non-canonical mutations were nonsense. In pts with persisting DNMT3A and ASXL1 aberrations, non-canonical mutations persisted at significantly lower variant allele frequencies (VAFs) compared to canonical mutations (P=.02, Figure 1A). Overall, the retained detection of DTA mutations did not associate with a higher cumulative incidence of relapse (CIR, P=.37) or overall survival (OS, P=.34) after HSCT. However, in DNMT3A or ASXL1 mutated pts, the persistence of a non-canonical mutation associated with significantly higher CIR (P=.01, Figure 1B) and shorter OS (P=.04) compared to the persistence of canonical mutations.
Conclusion
In general, the continuing detection of DTA mutations prior to HSCT did not associate with outcomes in AML pts transplanted in CR/CRi. In pts with DNMT3A or ASXL1 mutations, non-canonical mutations persisted at lower VAF levels than canonical mutations. The persistence of non-canonical ASXL1 or DNMT3A mutations associated with worse outcomes compared to the persistence of canonical mutations, challenging the paradigm that all DTA mutations are unsuitable as residual disease markers in AML.
Keyword(s): AML, Mutation status, Prognosis, Stem cell transplant
Abstract: EP435
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
In acute myeloid leukemia (AML), mutations in the genes DNMT3A, TET2, and ASXL1 (DTA) are frequent and appear early in leukemogenesis. In older, otherwise healthy individuals DTA mutations may be present as clonal hematopoiesis of indeterminate potential (CHIP). While TET2 mutations do not have a common hotspot, mutations in DNMT3A and ASXL1 frequently occur at specific positions (DNMT3A in R882 and ASXL1 as G646fs*12, referred to as “canonical”). The persistence of gene mutations prior to allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation therapy may identify AML patients (pts) at high-risk of relapse. Until today, no study genuinely evaluated the applicability of DTA mutations - including canonical vs. non-canonical mutations - as residual disease markers in AML pts receiving HSCT consolidation.
Aims
To evaluate DTA mutations as residual AML disease markers in pts prior to allogeneic HSCT.
Methods
We analyzed 68 AML pts harboring at least one DTA mutation (DNMT3A, n=41; TET2, n=18; ASXL1, n=15) at diagnosis, who received HSCT (median age 64, range 35-75 years) in complete remission (CR; n=50) or CR with incomplete recovery (CRi, n=18). Conditioning regimens were non-myeloablative (n=57), reduced intensity (n=7) or myeloablative (n=4). At diagnosis, the mutational status of 54 genes was evaluated using next generation sequencing at diagnosis. Applying highly sensitive mutation-specific digital droplet PCR (ddPCR) assays at the timepoint of HSCT, we tested for the persistence of DTA mutations. Median follow up was 5.0 years.
Results
At diagnosis, 41 pts had a DNMT3A mutation (62%; canonical: 61%; non-canonical: 39%) 18 had a TET2 mutation (33%) and 15 had an ASXL1 mutation (22%; canonical: 80%; non-canonical: 20%). Most frequently co-mutated genes were NPM1 (43%), RUNX1 (19%), IDH2 (16%), and BCOR, IDH1, and FLT3-ITD (each with 15%). At HSCT, DTA mutations persisted in the majority of pts (overall 84%; DNMT3A 86%, TET2 93%, and ASXL1 60%). The rate of sustained detection did not differ significantly between canonical and non-canonical mutations (DNMT3A: 89% vs 80%, P=1 and ASXL1: 57% vs 67%, P=1). Of the analyzed non-canonical mutations in DNMT3A 10% were frameshift, 60% were missense, and 30% were nonsense, while in ASXL1 all non-canonical mutations were nonsense. In pts with persisting DNMT3A and ASXL1 aberrations, non-canonical mutations persisted at significantly lower variant allele frequencies (VAFs) compared to canonical mutations (P=.02, Figure 1A). Overall, the retained detection of DTA mutations did not associate with a higher cumulative incidence of relapse (CIR, P=.37) or overall survival (OS, P=.34) after HSCT. However, in DNMT3A or ASXL1 mutated pts, the persistence of a non-canonical mutation associated with significantly higher CIR (P=.01, Figure 1B) and shorter OS (P=.04) compared to the persistence of canonical mutations.

Conclusion
In general, the continuing detection of DTA mutations prior to HSCT did not associate with outcomes in AML pts transplanted in CR/CRi. In pts with DNMT3A or ASXL1 mutations, non-canonical mutations persisted at lower VAF levels than canonical mutations. The persistence of non-canonical ASXL1 or DNMT3A mutations associated with worse outcomes compared to the persistence of canonical mutations, challenging the paradigm that all DTA mutations are unsuitable as residual disease markers in AML.
Keyword(s): AML, Mutation status, Prognosis, Stem cell transplant


