
Contributions
Abstract: EP433
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
All-trans retinoic acid (ATRA) and intravenous arsenic trioxide (IV ATO) cure most patients with acute promyelocytic leukemia (APL), but the requirement for 2-hourly ATO infusions over several months is problematic. Orally administered ATO has the potential to significantly reduce resource utilization and improve the therapeutic experience without compromising efficacy.
Aims
To characterize the bioavailability of encapsulated oral ATO (Phebra, Australia) in patients with APL by comparing the total arsenic AUC0-24 and Cmax in whole blood (WB) and plasma (PL) after oral and IV ATO administration.
Methods
The APML5 trial (ACTRN12616001022459) was a phase I, 2-part, 2-sequence, 4-period, bioavailability study in 31 consenting patients with previously untreated APL, embedded within a standard-of-care consolidation regimen (ATRA + IV ATO; Lo-Coco et al, NEJM 369:111,2013). Eligibility required documented hematological complete remission after induction with ATRA + IV ATO (+ idarubicin for high-risk APL). Initial pharmacokinetic (PK) data from the APML5 part 1 pilot (n=9) indicated oral and IV ATO provide comparable arsenic exposure over repeated cycles of consolidation (Iland et al, EHA 2019, HemaSphere doi:10.1097/01.HS9.0000562408.61553.8e).
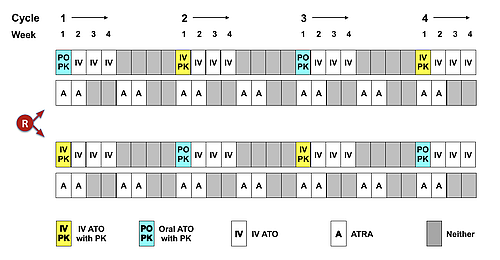
To conclusively establish bioequivalence, 22 patients at 12 sites were randomised in APML5 part 2 (Jan 2019-June 2020) to IV or oral ATO 0.15mg/kg/d with PK sampling in week 1 of cycles 1 & 3, and the alternate route in week 1 of cycles 2 & 4 (Figure). Total arsenic was quantitated by inductively coupled plasma mass spectrometry. Point estimates of mean oral/IV arsenic ratios ± 90% confidence intervals (CI) for AUC0-24 (μmol/l.h) and Cmax (μmol/l) in WB and PL were calculated by linear mixed model analysis incorporating fixed and random effects.
Results
The target accrual of 20 evaluable patients was reached after 2 replacements (insufficient PK data as per protocol). Median age was 45.9 years (range 23.4-75.2), 10 male, 10 female, 14 Sanz standard-risk, and 6 high-risk. Both ATO formulations were associated with significant increases in AUC0-24 and Cmax in WB and PL from day 1 to day 4 of each cycle (p<0.001), indicative of short-term arsenic accumulation, but no accumulation occurred between sequential cycles. Estimates of the geometric mean of the oral/IV ratio for each PK parameter closely approximate unity (Table), and the 90% CI fall within the conventional bioequivalence limits (0.80, 1.25). Distinguishing adverse events (AEs) specifically related to oral ATO from those related to IV ATO is problematic, since IV ATO was also administered for 3 weeks in cycles that commenced with 1 week of oral ATO (Figure). Nevertheless, no excess of grade 3-4 AEs (including gastrointestinal toxicity) was noted in cycles that contained oral ATO, and no instances of oral or IV ATO-associated QTc prolongation were observed during part 2 of APML5, despite monitoring with twice weekly electrocardiograms in each cycle. One patient with high-risk disease relapsed after consolidation.
PK parameter | Estimated geometric mean of oral/IV ratio | Lower limit (90% CI) | Upper limit (90% CI) |
WB AUC0-24 | 0.9931 | 0.9542 | 1.034 |
PL AUC0-24 | 1.030 | 0.9773 | 1.087 |
WB Cmax | 0.9174 | 0.8709 | 0.9663 |
PL Cmax | 0.9692 | 0.8923 | 1.053 |
Conclusion
This randomized crossover bioavailability study confirmed bioequivalence of the oral and IV formulations of ATO when administered in the context of standard-of-care consolidation, and unexpected toxicity signals were not evident. Overall, the results of the APML5 trial provide a sound rationale for incorporating oral ATO in the management of patients with APL.
Keyword(s): Acute promyelocytic leukemia, Arsenic trioxide, Oral, Pharmacokinetic
Abstract: EP433
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
All-trans retinoic acid (ATRA) and intravenous arsenic trioxide (IV ATO) cure most patients with acute promyelocytic leukemia (APL), but the requirement for 2-hourly ATO infusions over several months is problematic. Orally administered ATO has the potential to significantly reduce resource utilization and improve the therapeutic experience without compromising efficacy.
Aims
To characterize the bioavailability of encapsulated oral ATO (Phebra, Australia) in patients with APL by comparing the total arsenic AUC0-24 and Cmax in whole blood (WB) and plasma (PL) after oral and IV ATO administration.
Methods
The APML5 trial (ACTRN12616001022459) was a phase I, 2-part, 2-sequence, 4-period, bioavailability study in 31 consenting patients with previously untreated APL, embedded within a standard-of-care consolidation regimen (ATRA + IV ATO; Lo-Coco et al, NEJM 369:111,2013). Eligibility required documented hematological complete remission after induction with ATRA + IV ATO (+ idarubicin for high-risk APL). Initial pharmacokinetic (PK) data from the APML5 part 1 pilot (n=9) indicated oral and IV ATO provide comparable arsenic exposure over repeated cycles of consolidation (Iland et al, EHA 2019, HemaSphere doi:10.1097/01.HS9.0000562408.61553.8e).
To conclusively establish bioequivalence, 22 patients at 12 sites were randomised in APML5 part 2 (Jan 2019-June 2020) to IV or oral ATO 0.15mg/kg/d with PK sampling in week 1 of cycles 1 & 3, and the alternate route in week 1 of cycles 2 & 4 (Figure). Total arsenic was quantitated by inductively coupled plasma mass spectrometry. Point estimates of mean oral/IV arsenic ratios ± 90% confidence intervals (CI) for AUC0-24 (μmol/l.h) and Cmax (μmol/l) in WB and PL were calculated by linear mixed model analysis incorporating fixed and random effects.
Results
The target accrual of 20 evaluable patients was reached after 2 replacements (insufficient PK data as per protocol). Median age was 45.9 years (range 23.4-75.2), 10 male, 10 female, 14 Sanz standard-risk, and 6 high-risk. Both ATO formulations were associated with significant increases in AUC0-24 and Cmax in WB and PL from day 1 to day 4 of each cycle (p<0.001), indicative of short-term arsenic accumulation, but no accumulation occurred between sequential cycles. Estimates of the geometric mean of the oral/IV ratio for each PK parameter closely approximate unity (Table), and the 90% CI fall within the conventional bioequivalence limits (0.80, 1.25). Distinguishing adverse events (AEs) specifically related to oral ATO from those related to IV ATO is problematic, since IV ATO was also administered for 3 weeks in cycles that commenced with 1 week of oral ATO (Figure). Nevertheless, no excess of grade 3-4 AEs (including gastrointestinal toxicity) was noted in cycles that contained oral ATO, and no instances of oral or IV ATO-associated QTc prolongation were observed during part 2 of APML5, despite monitoring with twice weekly electrocardiograms in each cycle. One patient with high-risk disease relapsed after consolidation.
PK parameter | Estimated geometric mean of oral/IV ratio | Lower limit (90% CI) | Upper limit (90% CI) |
WB AUC0-24 | 0.9931 | 0.9542 | 1.034 |
PL AUC0-24 | 1.030 | 0.9773 | 1.087 |
WB Cmax | 0.9174 | 0.8709 | 0.9663 |
PL Cmax | 0.9692 | 0.8923 | 1.053 |

Conclusion
This randomized crossover bioavailability study confirmed bioequivalence of the oral and IV formulations of ATO when administered in the context of standard-of-care consolidation, and unexpected toxicity signals were not evident. Overall, the results of the APML5 trial provide a sound rationale for incorporating oral ATO in the management of patients with APL.
Keyword(s): Acute promyelocytic leukemia, Arsenic trioxide, Oral, Pharmacokinetic


