Contributions
Abstract: EP432
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Multiparameter flow cytometric assessment of measurable residual disease (MFC-MRD) in acute myeloid leukaemia (AML) has important prognostic value in predicting future relapse in patients who achieve a complete response to front-line chemotherapy. Efforts to harmonise MFC-MRD are ongoing but considerable expertise is still required for its analysis stage, with potential for variable interpretation and therefore prognostic value.
Aims
Prognostic evaluation of an unsupervised (Unsup) analysis method, developed to generate MFC-MRD results that are reproducible without reliance on significant expertise or diagnostic samples.
Methods
Comparison of the prognostic performance of our Unsup pipeline to conventional MFC-MRD analysis was performed, first for validation on post induction MFC-MRD samples and then on pre-transplantation samples from the FIGARO trial (Craddock et al, JCO 2020). Bone marrow samples (BM) were assessed in these trials for MFC-MRD as previously described (Freeman et al, JCO 2018). Phenograph clustering was performed on downsampled blast flow files in runs of up to 50 AML samples plus ~40 control BMs. Fluorescence intensity of markers on control blasts in each algorithm-defined cluster was used to statistically define the limits of normal marker expression per cluster. Blasts with aberrant expression defined by these cluster-specific reference range limits were quantified to produce Unsup-MRD results as a % of leukocytes.
Results
Results for assay validation were generated from 148 BMs after 1st and 2nd induction from 111 patients (39 CBF, 44 NPM-1mut and 28 Flt3-ITD+NPM1wt AMLs).
Unsup-MRD values were higher than standard MFC-MRD (which estimates %MRD from a single leukemic aberrant immunophenotype) but correlated positively. Polynomial regression showed 0.32% Unsup-MRD to have equivalence to the ELN recommended cut-off of 0.1% for standard MFC-MRD positivity. Duplicate testing demonstrated result repeatability (r2=0.876; p<0.001). Minimum analytical sensitivity determined from clustering runs of control BMs tested against reference control BMs was 0.13% for the limit of blank / 0.20% for the limit of detection. From in silico spiking of AML blasts into control blasts, mean recovery was 84-108% with Unsup-MRD detected at >5% of blasts at the lowest dilution.
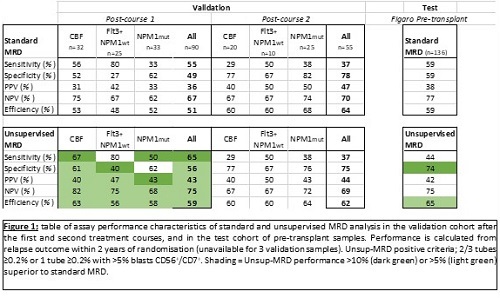
To optimise test specificity, Unsup-MRD positivity criteria were set at ≥0.2% in ≥2 tubes unless CD56+/ CD7+ at ≥0.2%. Importantly, assay statistics for relapse within 2yrs were highly similar between Unsup-MRD and standard MRD in the test cohort (Figure 1). Of note, the negative predictive value of Unsup-MRD post-C1 was 82% and 75% for CBF and Flt3-ITD+NPM1wt patients respectively.
We then applied the validated Unsup-MRD criteria to the pre-transplant BMs from 136 FIGARO patients. Assay performance was again comparable to standard MRD (Figure 1). 2yr cumulative incidence of relapse (CIR) was 22.2% for MRDneg vs 42.1% for MRDpos by Unsup criteria (p=0.03).
We next tested the higher cut-off (≥0.3%) equivalent to 0.1% standard MFC-MRD. This had little impact on test efficiency for the post-induction validation cohort but increased pre-transplant Unsup-MRD specificity (82% from 71%) with a 2yr CIR of 21.2% MRDneg vs 51.9% MRDpos (p<0.001). These results support previous observations that relapse risk after reduced intensity allogeneic transplant. may not be significantly increased when MFC-MRD is detected at low levels pre-transplant.
Conclusion
The prognostic performance of a novel unsupervised approach for assessing AML MFC-MRD is at least equivalent to standard MFC-MRD analysis.
Keyword(s): AML, Flow cytometry, MRD, Transplant
Abstract: EP432
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Multiparameter flow cytometric assessment of measurable residual disease (MFC-MRD) in acute myeloid leukaemia (AML) has important prognostic value in predicting future relapse in patients who achieve a complete response to front-line chemotherapy. Efforts to harmonise MFC-MRD are ongoing but considerable expertise is still required for its analysis stage, with potential for variable interpretation and therefore prognostic value.
Aims
Prognostic evaluation of an unsupervised (Unsup) analysis method, developed to generate MFC-MRD results that are reproducible without reliance on significant expertise or diagnostic samples.
Methods
Comparison of the prognostic performance of our Unsup pipeline to conventional MFC-MRD analysis was performed, first for validation on post induction MFC-MRD samples and then on pre-transplantation samples from the FIGARO trial (Craddock et al, JCO 2020). Bone marrow samples (BM) were assessed in these trials for MFC-MRD as previously described (Freeman et al, JCO 2018). Phenograph clustering was performed on downsampled blast flow files in runs of up to 50 AML samples plus ~40 control BMs. Fluorescence intensity of markers on control blasts in each algorithm-defined cluster was used to statistically define the limits of normal marker expression per cluster. Blasts with aberrant expression defined by these cluster-specific reference range limits were quantified to produce Unsup-MRD results as a % of leukocytes.
Results
Results for assay validation were generated from 148 BMs after 1st and 2nd induction from 111 patients (39 CBF, 44 NPM-1mut and 28 Flt3-ITD+NPM1wt AMLs).
Unsup-MRD values were higher than standard MFC-MRD (which estimates %MRD from a single leukemic aberrant immunophenotype) but correlated positively. Polynomial regression showed 0.32% Unsup-MRD to have equivalence to the ELN recommended cut-off of 0.1% for standard MFC-MRD positivity. Duplicate testing demonstrated result repeatability (r2=0.876; p<0.001). Minimum analytical sensitivity determined from clustering runs of control BMs tested against reference control BMs was 0.13% for the limit of blank / 0.20% for the limit of detection. From in silico spiking of AML blasts into control blasts, mean recovery was 84-108% with Unsup-MRD detected at >5% of blasts at the lowest dilution.
To optimise test specificity, Unsup-MRD positivity criteria were set at ≥0.2% in ≥2 tubes unless CD56+/ CD7+ at ≥0.2%. Importantly, assay statistics for relapse within 2yrs were highly similar between Unsup-MRD and standard MRD in the test cohort (Figure 1). Of note, the negative predictive value of Unsup-MRD post-C1 was 82% and 75% for CBF and Flt3-ITD+NPM1wt patients respectively.
We then applied the validated Unsup-MRD criteria to the pre-transplant BMs from 136 FIGARO patients. Assay performance was again comparable to standard MRD (Figure 1). 2yr cumulative incidence of relapse (CIR) was 22.2% for MRDneg vs 42.1% for MRDpos by Unsup criteria (p=0.03).
We next tested the higher cut-off (≥0.3%) equivalent to 0.1% standard MFC-MRD. This had little impact on test efficiency for the post-induction validation cohort but increased pre-transplant Unsup-MRD specificity (82% from 71%) with a 2yr CIR of 21.2% MRDneg vs 51.9% MRDpos (p<0.001). These results support previous observations that relapse risk after reduced intensity allogeneic transplant. may not be significantly increased when MFC-MRD is detected at low levels pre-transplant.
Conclusion
The prognostic performance of a novel unsupervised approach for assessing AML MFC-MRD is at least equivalent to standard MFC-MRD analysis.
Keyword(s): AML, Flow cytometry, MRD, Transplant