
Contributions
Abstract: EP431
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Secondary acute myeloid leukemia (sAML) has been associated with inferior outcomes compared to de novo AML. Relapse (rel) is the most frequent cause of transplant failure reaching up to 50% in high-risk AML. Median OS in sAML is 8 months. The available data for patients (pts) with sAML undergoing a second hematopoietic stem cell transplantation (HSCT2) for rel post first transplantation (HSCT1) is limited. Furthermore, no study has compared myeloablative (MAC) versus reduced intensity conditioning (RIC) for HSCT2 in sAML.
Aims
The study aimed to assess HSCT2 outcome for sAML comparing MAC to RIC.
Methods
This was a retrospective analysis based on data from the EBMT registry, including adult pts aged ≥18 years with sAML undergoing HSCT2 during the years 2005-2019 for rel post-HSCT1. Allografts were from a matched sibling donor (MSD), unrelated donor (UD), or haploidentical donor (Haplo) for both HSCT1 and HSCT2. Multivariate analysis (MVA) adjusting for potential confounding factors was performed using a Cox’s proportional-hazards regression model for main outcomes.
Results
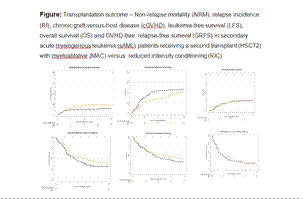
215 pts were included: RIC (n=134), MAC (n=81). Median F/U was 41.0 (27.7-69.3) and 28.5 (23.9-75.4) months. Median age was 55.1 (23.9-70.1) and 52.5 (18.2-67.8) years (p=0.10), and 56.0% and 54.3% were male (p=0.81), respectively. Median time from HSCT1 to rel for MAC and RIC was 19 and 24 months (p=0.55). Disease status was CR in 40.3% and 40.8%, and active disease in 59.7% and 59.2% (p=0.95), respectively. KPS was >90 in 55.8% and 56.7% of pts, respectively (p=0.90). Donors were MSD in 24.6% and 25.9%, UD in 61.2% and 56.8%, and Haplo in 14.2 and 17.3%, respectively (p=0.77). Graft was peripheral blood in 94.8% and 88.9% of transplants (p=0.11) and TBI was administered to 20.1% and 27.2%, (p=0.24), respectively. The most frequent RIC was Fludarabine/ Busulfan (Flu/Bu)-13.2%, Flu/TBI-10.1%, and Flu/Melphalan (Mel)-9.3%, while the most frequent MAC was Flu/Bu-9%, Flu/Thiotepa (Thio)/Treosulfan (Treo)-9%, and Flu/TBI-6.4%. In vivo T-cell depletion with antithymocyte globulin (ATG) was used in 51.3% and 55.6 % of transplants (p=0.56). Graft-versus-host disease (GVHD) prophylaxis was cyclosporine A (CSA)/mycophenolate mofetil + ATG in 31.9 % and 34.7%, and CSA/methotrexate + ATG in 15.9% and 19.4% of the pts. At 2-years the rel incidence was 58.3% vs 51.1% in MAC and RIC, respectively (p=0.24). The 2-y LFS was 26.6% vs 26%, (p=0.55), and the GRFS was 16.4% vs 12.1% (p=0.88), while OS was 31.4% and 39.7%, (p=0.41), respectively. 68% and 61.7% of pts receiving MAC and RIC died .Main cause of death was original disease 58.4% and 40.8% followed by infections and GVHD 10.1% and 10.2%; and 6.7% and 4.1%, respectively. MVA showed a significantly lower rel and improved LFS with MAC vs RIC, HR=0.46 (95% CI, 0.26-0.8, p=0.006) vs HR=0.62 (95% CI, 0.39-0.98, p=0.042), respectively, while OS, HR=0.72 (95% CI, 0.44-1. 17, p=0.18) and GRFS, HR=0.89 (95% CI, 0.59-1.36, p=0.6), respectively, did not differ significantly. Other significant prognostic factors for HSCT2 outcome were disease status, time from HSCT1 to relapse, and KPS.
Conclusion
In this EBMT registry study of HSCT2 for sAML, MAC was associated with a lower relapse risk and superior LFS. Disease status, time from HSCT1 to relapse, and KPS are additional prognostic factors for transplantation outcome. These results are consistent with our previously reported findings in pts with sAML undergoing HSCT1 (Blood Advances 2:2127-2135, 2018), in favor of MAC for pts with sAML who received an HSCT2 after relapse.
Keyword(s): Acute myeloid leukemia, Adult, Allogeneic hematopoietic stem cell transplant, Conditioning
Abstract: EP431
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Secondary acute myeloid leukemia (sAML) has been associated with inferior outcomes compared to de novo AML. Relapse (rel) is the most frequent cause of transplant failure reaching up to 50% in high-risk AML. Median OS in sAML is 8 months. The available data for patients (pts) with sAML undergoing a second hematopoietic stem cell transplantation (HSCT2) for rel post first transplantation (HSCT1) is limited. Furthermore, no study has compared myeloablative (MAC) versus reduced intensity conditioning (RIC) for HSCT2 in sAML.
Aims
The study aimed to assess HSCT2 outcome for sAML comparing MAC to RIC.
Methods
This was a retrospective analysis based on data from the EBMT registry, including adult pts aged ≥18 years with sAML undergoing HSCT2 during the years 2005-2019 for rel post-HSCT1. Allografts were from a matched sibling donor (MSD), unrelated donor (UD), or haploidentical donor (Haplo) for both HSCT1 and HSCT2. Multivariate analysis (MVA) adjusting for potential confounding factors was performed using a Cox’s proportional-hazards regression model for main outcomes.
Results
215 pts were included: RIC (n=134), MAC (n=81). Median F/U was 41.0 (27.7-69.3) and 28.5 (23.9-75.4) months. Median age was 55.1 (23.9-70.1) and 52.5 (18.2-67.8) years (p=0.10), and 56.0% and 54.3% were male (p=0.81), respectively. Median time from HSCT1 to rel for MAC and RIC was 19 and 24 months (p=0.55). Disease status was CR in 40.3% and 40.8%, and active disease in 59.7% and 59.2% (p=0.95), respectively. KPS was >90 in 55.8% and 56.7% of pts, respectively (p=0.90). Donors were MSD in 24.6% and 25.9%, UD in 61.2% and 56.8%, and Haplo in 14.2 and 17.3%, respectively (p=0.77). Graft was peripheral blood in 94.8% and 88.9% of transplants (p=0.11) and TBI was administered to 20.1% and 27.2%, (p=0.24), respectively. The most frequent RIC was Fludarabine/ Busulfan (Flu/Bu)-13.2%, Flu/TBI-10.1%, and Flu/Melphalan (Mel)-9.3%, while the most frequent MAC was Flu/Bu-9%, Flu/Thiotepa (Thio)/Treosulfan (Treo)-9%, and Flu/TBI-6.4%. In vivo T-cell depletion with antithymocyte globulin (ATG) was used in 51.3% and 55.6 % of transplants (p=0.56). Graft-versus-host disease (GVHD) prophylaxis was cyclosporine A (CSA)/mycophenolate mofetil + ATG in 31.9 % and 34.7%, and CSA/methotrexate + ATG in 15.9% and 19.4% of the pts. At 2-years the rel incidence was 58.3% vs 51.1% in MAC and RIC, respectively (p=0.24). The 2-y LFS was 26.6% vs 26%, (p=0.55), and the GRFS was 16.4% vs 12.1% (p=0.88), while OS was 31.4% and 39.7%, (p=0.41), respectively. 68% and 61.7% of pts receiving MAC and RIC died .Main cause of death was original disease 58.4% and 40.8% followed by infections and GVHD 10.1% and 10.2%; and 6.7% and 4.1%, respectively. MVA showed a significantly lower rel and improved LFS with MAC vs RIC, HR=0.46 (95% CI, 0.26-0.8, p=0.006) vs HR=0.62 (95% CI, 0.39-0.98, p=0.042), respectively, while OS, HR=0.72 (95% CI, 0.44-1. 17, p=0.18) and GRFS, HR=0.89 (95% CI, 0.59-1.36, p=0.6), respectively, did not differ significantly. Other significant prognostic factors for HSCT2 outcome were disease status, time from HSCT1 to relapse, and KPS.

Conclusion
In this EBMT registry study of HSCT2 for sAML, MAC was associated with a lower relapse risk and superior LFS. Disease status, time from HSCT1 to relapse, and KPS are additional prognostic factors for transplantation outcome. These results are consistent with our previously reported findings in pts with sAML undergoing HSCT1 (Blood Advances 2:2127-2135, 2018), in favor of MAC for pts with sAML who received an HSCT2 after relapse.
Keyword(s): Acute myeloid leukemia, Adult, Allogeneic hematopoietic stem cell transplant, Conditioning


