
Contributions
Abstract: EP430
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
The outcomes in patients (pts) with newly diagnosed FLT3 mutated (m) acute myeloid leukemia (AML) who are ineligible for intensive induction chemotherapy (IC) are poor. Added to a low-intensity chemotherapy backbone, first-generation FLT3 inhibitors (FLT3i), such as midostaurin and sorafenib, result in a median OS of 8 months in the frontline (Gallogly ASH 2017, Ohanian AJH 2018), and 4-8 months in relapsed/refractory (R/R) settings (Yilmaz JHO 2020, Ravandi Blood 2013). Quizartinib, a potent second-generation FLT3i, demonstrated synergy with venetoclax (VEN) in AML cell lines and PDX models (Mali Haematologica 2020). We designed this study to evaluate the safety and efficacy of quizartinib, VEN, and decitabine (DAC) combination in pts with R/R or newly diagnosed FLT3m AML.
Aims
To establish recommended phase 2 dose (RP2D) and optimal schedule of DAC + quizartinib + VEN in R/R or newly diagnosed FLT3m AML.
Methods
Frontline cohort included pts who are ineligible for IC, and R/R cohort included pts who received ≤ 5 prior treatments. All pts had a performance status of ECOG ≤2, QTcF <450 msec prior to therapy. All pts underwent day 14 bone marrow, and VEN (400 mg/day) was put on hold in patients with bone marrow (BM) blasts ≤ 5% (or marrow aplasia). Pts with day14 BM blast >5% continued venetoclax for 21 days during cycle 1. All pts induced with 10 days of DAC (20 mg/m2). In subsequent cycles, DAC was administered for 5 days. Quizartinib (30 or 40 mg/day) was administered daily continuously.
Results
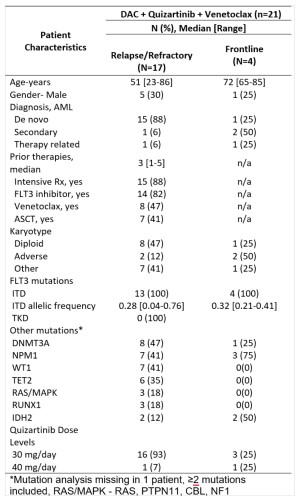
Overall 21 pts were enrolled and 17 evaluable at the time of this report (4 are still in cycle 1) (Table). Of 13 pts with R/R AML (median 3 [range 1-5] prior therapies, 85% with ≥1 prior FLT3i’s), 9 (69%) achieved CRc (2 CR, 7 CRi) with 4/9 and 5/9 evaluable responders FLT3-PCR (sensitivity 10-2) and multicolor flow cytometry (MFC) negativity (sensitivity 10-4), respectively. The median number of cycles to response was 1 [range 1-2]. 30- and 60-day mortality rates were 0% and 8%. Of 4 patients with newly diagnosed AML, all achieved CRc (2 CR, 2 CRi) with 4/4 and 2/3 evaluable responders negative MRD by FLT3-PCR and MFC, respectively. No pts developed a dose-limiting toxicity (DLT) with 30 mg/day quizartinib, however with the 40mg/day quizartinib, the first 2 patients treated developed hematologic DLT (grade ≥3 neutropenia with a <5% cellular bone marrow with no residual AML lasting ≥42 days). Hence, quizartinib 30 mg/day dose was declared RP2D for the triplet. Grade ≥3 non-hematologic toxicities in >2 pts included lung infections (N=9) and neutropenic fever (N=6). No unexpected toxicities were noted. No QTcF prolongations >480 msec were noted. With a median follow-up of 7.2 months, the median OS was not reached in the frontline cohort and was 7.1 months in R/R cohort . 2/4 and 5/9 CRc pts underwent ASCT in frontline and R/R cohorts, respectively. All frontline patients were alive at the last follow-up; 3 in CR and 1 relapsed disease on salvage therapy. Of 9 CRc pts in the R/R cohort, 4 are alive (3 CR, 1 relapse), and 5 died (4 from relapse, 1 death in CR from sepsis). Baseline and longitudinal 36-marker mass cytometry (CYTOF), including FLT3, STAT, RAS-MAPK, and apoptosis pathway markers, is ongoing.
Conclusion
DAC + VEN + quizartinib is active in heavily pretreated and prior FLT3i exposed R/R FLT3-ITDm pts, with CRc rates of 69% (55% of responders were negative MRD by MFC) and the med OS of 7.1 months. The regimen was well tolerated with no 30-day mortality. Accrual to the triplet continues and updated clinical and correlative data will be presented.
Keyword(s): AML, Flt3 inhibitor
Abstract: EP430
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
The outcomes in patients (pts) with newly diagnosed FLT3 mutated (m) acute myeloid leukemia (AML) who are ineligible for intensive induction chemotherapy (IC) are poor. Added to a low-intensity chemotherapy backbone, first-generation FLT3 inhibitors (FLT3i), such as midostaurin and sorafenib, result in a median OS of 8 months in the frontline (Gallogly ASH 2017, Ohanian AJH 2018), and 4-8 months in relapsed/refractory (R/R) settings (Yilmaz JHO 2020, Ravandi Blood 2013). Quizartinib, a potent second-generation FLT3i, demonstrated synergy with venetoclax (VEN) in AML cell lines and PDX models (Mali Haematologica 2020). We designed this study to evaluate the safety and efficacy of quizartinib, VEN, and decitabine (DAC) combination in pts with R/R or newly diagnosed FLT3m AML.
Aims
To establish recommended phase 2 dose (RP2D) and optimal schedule of DAC + quizartinib + VEN in R/R or newly diagnosed FLT3m AML.
Methods
Frontline cohort included pts who are ineligible for IC, and R/R cohort included pts who received ≤ 5 prior treatments. All pts had a performance status of ECOG ≤2, QTcF <450 msec prior to therapy. All pts underwent day 14 bone marrow, and VEN (400 mg/day) was put on hold in patients with bone marrow (BM) blasts ≤ 5% (or marrow aplasia). Pts with day14 BM blast >5% continued venetoclax for 21 days during cycle 1. All pts induced with 10 days of DAC (20 mg/m2). In subsequent cycles, DAC was administered for 5 days. Quizartinib (30 or 40 mg/day) was administered daily continuously.
Results
Overall 21 pts were enrolled and 17 evaluable at the time of this report (4 are still in cycle 1) (Table). Of 13 pts with R/R AML (median 3 [range 1-5] prior therapies, 85% with ≥1 prior FLT3i’s), 9 (69%) achieved CRc (2 CR, 7 CRi) with 4/9 and 5/9 evaluable responders FLT3-PCR (sensitivity 10-2) and multicolor flow cytometry (MFC) negativity (sensitivity 10-4), respectively. The median number of cycles to response was 1 [range 1-2]. 30- and 60-day mortality rates were 0% and 8%. Of 4 patients with newly diagnosed AML, all achieved CRc (2 CR, 2 CRi) with 4/4 and 2/3 evaluable responders negative MRD by FLT3-PCR and MFC, respectively. No pts developed a dose-limiting toxicity (DLT) with 30 mg/day quizartinib, however with the 40mg/day quizartinib, the first 2 patients treated developed hematologic DLT (grade ≥3 neutropenia with a <5% cellular bone marrow with no residual AML lasting ≥42 days). Hence, quizartinib 30 mg/day dose was declared RP2D for the triplet. Grade ≥3 non-hematologic toxicities in >2 pts included lung infections (N=9) and neutropenic fever (N=6). No unexpected toxicities were noted. No QTcF prolongations >480 msec were noted. With a median follow-up of 7.2 months, the median OS was not reached in the frontline cohort and was 7.1 months in R/R cohort . 2/4 and 5/9 CRc pts underwent ASCT in frontline and R/R cohorts, respectively. All frontline patients were alive at the last follow-up; 3 in CR and 1 relapsed disease on salvage therapy. Of 9 CRc pts in the R/R cohort, 4 are alive (3 CR, 1 relapse), and 5 died (4 from relapse, 1 death in CR from sepsis). Baseline and longitudinal 36-marker mass cytometry (CYTOF), including FLT3, STAT, RAS-MAPK, and apoptosis pathway markers, is ongoing.

Conclusion
DAC + VEN + quizartinib is active in heavily pretreated and prior FLT3i exposed R/R FLT3-ITDm pts, with CRc rates of 69% (55% of responders were negative MRD by MFC) and the med OS of 7.1 months. The regimen was well tolerated with no 30-day mortality. Accrual to the triplet continues and updated clinical and correlative data will be presented.
Keyword(s): AML, Flt3 inhibitor


