
Contributions
Abstract: EP429
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Phase 3 studies have confirmed the benefit of adding venetoclax (VEN) to azacitidine (AZA) or low dose cytarabine (LDAC) in older/unfit patients (pts) with newly diagnosed AML. For pts responding to VEN-AZA or VEN-LDAC, the median duration of response was 18 and 12 months, respectively. Therefore, remission duration will exceed 12 months in majority of responders. It is currently unknown whether pts should continue therapy until progression and whether elective cessation of therapy could detrimentally impact long-term outcome.
Aims
To compare the natural history of pts receiving ≥12 months of VEN-based therapy in first remission after electively ceasing therapy or continuing treatment until progression.
Methods
Pts with AML receiving VEN combined with either hypomethylating agents (HMA) or LDAC for ≥12 months and in first remission were included in this retrospective analysis. Two treatment approaches were compared: elective cessation of therapy in remission followed by monitoring (STOP cohort) or continued therapy until relapse (CONT cohort). Data cut-off was 31Jan2021.
Results
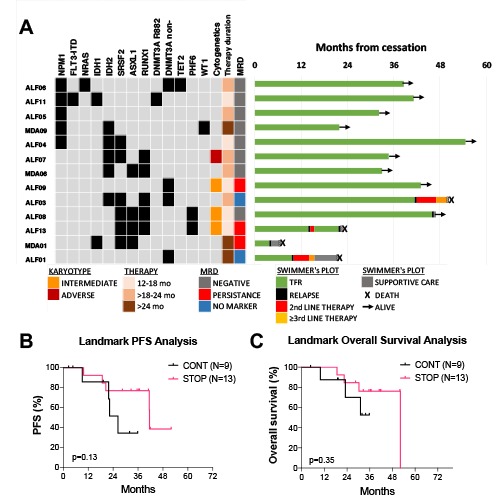
25 pts from 2 centres were included: 13 in STOP cohort and 12 in CONT cohort. The median follow-up was 55 months. Median age at AML diagnosis was 74 years (range 65-80). Treatments included VEN-HMA (68%) or VEN-LDAC (32%). Both STOP and CONT cohorts had comparable rates of ELN 2017 adverse risk (50% vs 46%). The median number of cycles received was shorter in the STOP (17; range 12-29) vs CONT (30; range 12-57) cohort. Median time on therapy (TOT) was also shorter in the STOP cohort (19.1 vs 33.2 months). In the STOP group, the reason for treatment cessation was patient request in 46% and medical in 54%. After ceasing therapy in the STOP cohort, the median treatment-free remission (TFR) was 45.8 months (range 3.8-54.5)(Figure 1A). Five pts (38%) subsequently relapsed, of which 3 had concurrent SRSF2 and ASXL1mutations. 2/5 relapses occurred >36 months of TFR. In the CONT cohort, 5 pts were receiving ongoing therapy at data cut-off. Eight (67%) have relapsed, with 5 relapsing >24 months of therapy. More pts had acquisition of cytogenetic abnormalities at relapse in the CONT cohort (6/8; 75%), compared to the STOP group (2/5; 40%).
Overall, 14 pts had NPM1 and/or IDH2 mutations (mut): 8 in the STOP and 6 in CONT cohort. 10 achieved undetectable MRD (by flow cytometry and/or qPCR), of which 1 patient (10%) relapsed. In the STOP cohort, 7/8 with NPM1 and/or IDH2 mut are still in TFR. We next performed a landmark analysis to compare OS among pts in the STOP and CONT cohorts. The landmark was taken from the median TOT in the STOP group (19 months), in order to exclude lead-time bias in pts relapsing early in the CONT group. This revealed no significant difference in progression free (p=0.13, Figure 1B) or overall survival (p=0.35, Figure 1C) between the two groups. Among relapsed pts, 3/5 in the STOP and 4/8 in CONT cohorts were salvaged. In the STOP group, 2/3 relapsing pts re-challenged with the same VEN-based regimen achieved second CR/CRi. The median time from relapse to death was similar in both groups: median 9.8 vs 6 months in the STOP vs CONT cohorts, respectively (p=0.15).
Conclusion
In elderly/unfit pts with AML receiving front line VEN-based treatment and in CR/CRi after 12 months therapy, the likelihood of a durable TFR is highest among pts with NPM1/IDH2 mutation and MRD negativity at the time of therapy cessation. A prospective, randomised, discontinuation study in pts in CRMRDneg after 12 months should be considered.
Keyword(s): AML, BCL2, Treatment-free remission
Abstract: EP429
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Phase 3 studies have confirmed the benefit of adding venetoclax (VEN) to azacitidine (AZA) or low dose cytarabine (LDAC) in older/unfit patients (pts) with newly diagnosed AML. For pts responding to VEN-AZA or VEN-LDAC, the median duration of response was 18 and 12 months, respectively. Therefore, remission duration will exceed 12 months in majority of responders. It is currently unknown whether pts should continue therapy until progression and whether elective cessation of therapy could detrimentally impact long-term outcome.
Aims
To compare the natural history of pts receiving ≥12 months of VEN-based therapy in first remission after electively ceasing therapy or continuing treatment until progression.
Methods
Pts with AML receiving VEN combined with either hypomethylating agents (HMA) or LDAC for ≥12 months and in first remission were included in this retrospective analysis. Two treatment approaches were compared: elective cessation of therapy in remission followed by monitoring (STOP cohort) or continued therapy until relapse (CONT cohort). Data cut-off was 31Jan2021.
Results
25 pts from 2 centres were included: 13 in STOP cohort and 12 in CONT cohort. The median follow-up was 55 months. Median age at AML diagnosis was 74 years (range 65-80). Treatments included VEN-HMA (68%) or VEN-LDAC (32%). Both STOP and CONT cohorts had comparable rates of ELN 2017 adverse risk (50% vs 46%). The median number of cycles received was shorter in the STOP (17; range 12-29) vs CONT (30; range 12-57) cohort. Median time on therapy (TOT) was also shorter in the STOP cohort (19.1 vs 33.2 months). In the STOP group, the reason for treatment cessation was patient request in 46% and medical in 54%. After ceasing therapy in the STOP cohort, the median treatment-free remission (TFR) was 45.8 months (range 3.8-54.5)(Figure 1A). Five pts (38%) subsequently relapsed, of which 3 had concurrent SRSF2 and ASXL1mutations. 2/5 relapses occurred >36 months of TFR. In the CONT cohort, 5 pts were receiving ongoing therapy at data cut-off. Eight (67%) have relapsed, with 5 relapsing >24 months of therapy. More pts had acquisition of cytogenetic abnormalities at relapse in the CONT cohort (6/8; 75%), compared to the STOP group (2/5; 40%).
Overall, 14 pts had NPM1 and/or IDH2 mutations (mut): 8 in the STOP and 6 in CONT cohort. 10 achieved undetectable MRD (by flow cytometry and/or qPCR), of which 1 patient (10%) relapsed. In the STOP cohort, 7/8 with NPM1 and/or IDH2 mut are still in TFR. We next performed a landmark analysis to compare OS among pts in the STOP and CONT cohorts. The landmark was taken from the median TOT in the STOP group (19 months), in order to exclude lead-time bias in pts relapsing early in the CONT group. This revealed no significant difference in progression free (p=0.13, Figure 1B) or overall survival (p=0.35, Figure 1C) between the two groups. Among relapsed pts, 3/5 in the STOP and 4/8 in CONT cohorts were salvaged. In the STOP group, 2/3 relapsing pts re-challenged with the same VEN-based regimen achieved second CR/CRi. The median time from relapse to death was similar in both groups: median 9.8 vs 6 months in the STOP vs CONT cohorts, respectively (p=0.15).

Conclusion
In elderly/unfit pts with AML receiving front line VEN-based treatment and in CR/CRi after 12 months therapy, the likelihood of a durable TFR is highest among pts with NPM1/IDH2 mutation and MRD negativity at the time of therapy cessation. A prospective, randomised, discontinuation study in pts in CRMRDneg after 12 months should be considered.
Keyword(s): AML, BCL2, Treatment-free remission


