
Contributions
Abstract: EP428
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Demographic and disease factors influence survival outcomes for patients (pts) with AML. In the phase 3 QUAZAR AML-001 trial, Oral-AZA significantly prolonged overall (OS) and relapse-free (RFS) vs. placebo (PBO) for pts with AML in first remission after intensive chemotherapy (IC) (Wei, NEJM, 2020). Univariate analyses showed OS and RFS benefits with Oral-AZA vs. PBO across pt subgroups defined by baseline characteristics.
Aims
Multivariate analyses were performed to identify baseline characteristics independently predictive of OS and RFS in the QUAZAR AML-001 trial, and to assess Tx effects of Oral-AZA vs. PBO on OS and RFS when adjusted for baseline factors.
Methods
Pts were aged ≥ 55 yrs with AML in complete remission (CR) or CR with incomplete blood count recovery (CRi) after induction ± consolidation. Within 4 months of attaining CR/CRi, pts were randomized 1:1 to receive Oral-AZA 300 mg or PBO for 14d of repeated 28d cycles. Cox proportional hazards models were used to estimate the Tx effects of Oral-AZA vs. PBO on OS and RFS, adjusting for baseline age, sex, ECOG PS score, cytogenetic risk at diagnosis, prior myelodysplastic syndrome (MDS), geographic region, response after induction (CR or CRi per investigator) and at baseline (per sponsor), measurable residual disease (MRD) status, receipt of consolidation therapy, number of consolidation cycles, baseline platelet count, and baseline ANC. In a stepwise procedure, randomized Tx and baseline variables were selected incrementally into a Cox model if P ≤ 0.25. After each addition, the contribution of the covariate, adjusted for other covariates in the model, was evaluated and retained in the model if P ≤ 0.15.
Results
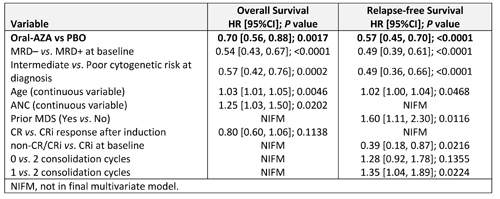
Oral-AZA Tx remained a significant independent predictor of improved OS (hazard ratio [HR] 0.70; P = 0.0017) and RFS (HR 0.57; P < 0.0001) vs. PBO after controlling for baseline characteristics (Table). MRD status at baseline, cytogenetic risk at diagnosis, and pt age were each also independently predictive of OS and RFS (Table). Response after induction (CR vs. CRi) and baseline ANC were predictive of OS but not RFS, whereas prior MDS, non-CR/CRi vs. CRi at baseline, and number of consolidation cycles were predictive only of RFS.
Conclusion
Tx with Oral-AZA reduced the risk of death by 30% and the risk of relapse by 43% vs. PBO, independent of baseline characteristics. Cytogenetic risk at diagnosis, MRD status at baseline, and pt age also independently predicted OS and RFS outcomes. CR vs. CRi response after induction, and no consolidation vs 2 consolidation cycles, did not significantly influence OS or RFS outcomes.
Keyword(s): AML, Azacitidine, Oral, Survival
Abstract: EP428
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Demographic and disease factors influence survival outcomes for patients (pts) with AML. In the phase 3 QUAZAR AML-001 trial, Oral-AZA significantly prolonged overall (OS) and relapse-free (RFS) vs. placebo (PBO) for pts with AML in first remission after intensive chemotherapy (IC) (Wei, NEJM, 2020). Univariate analyses showed OS and RFS benefits with Oral-AZA vs. PBO across pt subgroups defined by baseline characteristics.
Aims
Multivariate analyses were performed to identify baseline characteristics independently predictive of OS and RFS in the QUAZAR AML-001 trial, and to assess Tx effects of Oral-AZA vs. PBO on OS and RFS when adjusted for baseline factors.
Methods
Pts were aged ≥ 55 yrs with AML in complete remission (CR) or CR with incomplete blood count recovery (CRi) after induction ± consolidation. Within 4 months of attaining CR/CRi, pts were randomized 1:1 to receive Oral-AZA 300 mg or PBO for 14d of repeated 28d cycles. Cox proportional hazards models were used to estimate the Tx effects of Oral-AZA vs. PBO on OS and RFS, adjusting for baseline age, sex, ECOG PS score, cytogenetic risk at diagnosis, prior myelodysplastic syndrome (MDS), geographic region, response after induction (CR or CRi per investigator) and at baseline (per sponsor), measurable residual disease (MRD) status, receipt of consolidation therapy, number of consolidation cycles, baseline platelet count, and baseline ANC. In a stepwise procedure, randomized Tx and baseline variables were selected incrementally into a Cox model if P ≤ 0.25. After each addition, the contribution of the covariate, adjusted for other covariates in the model, was evaluated and retained in the model if P ≤ 0.15.
Results
Oral-AZA Tx remained a significant independent predictor of improved OS (hazard ratio [HR] 0.70; P = 0.0017) and RFS (HR 0.57; P < 0.0001) vs. PBO after controlling for baseline characteristics (Table). MRD status at baseline, cytogenetic risk at diagnosis, and pt age were each also independently predictive of OS and RFS (Table). Response after induction (CR vs. CRi) and baseline ANC were predictive of OS but not RFS, whereas prior MDS, non-CR/CRi vs. CRi at baseline, and number of consolidation cycles were predictive only of RFS.

Conclusion
Tx with Oral-AZA reduced the risk of death by 30% and the risk of relapse by 43% vs. PBO, independent of baseline characteristics. Cytogenetic risk at diagnosis, MRD status at baseline, and pt age also independently predicted OS and RFS outcomes. CR vs. CRi response after induction, and no consolidation vs 2 consolidation cycles, did not significantly influence OS or RFS outcomes.
Keyword(s): AML, Azacitidine, Oral, Survival


