
Contributions
Abstract: EP427
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematologic malignancy. CD123, the interleukin-3 (IL3) receptor alpha subunit, is overexpressed in BPDCN cells, making it an attractive therapeutic target. Tagraxofusp (TAG, SL-401) is a CD123-directed immunotherapy consisting of recombinant human IL3 and a truncated diphtheria toxin. TAG was FDA approved in 2018 for BPDCN treatment in adult and pediatric (≥2 y) patients (pts) and was recently approved in the EU as monotherapy for first line (1L) treatment in adults. Herein, we show long-term follow-up results from the largest prospective trial (NCT02113982) in pts with BPDCN.
Aims
To study the long-term safety and efficacy of TAG in pts with BPDCN.
Methods
This multicenter, open-label, phase 1/2 study consisted of Stages 1–4 (dose escalation, expansion, confirmatory, and continued access, respectively). Pts received TAG by IV infusion once daily on d 1–5 of a 21-d cycle; the dose was 7 or 12 mcg/kg in Stage 1 and 12 mcg/kg in Stages 2–4. The primary outcome was complete response (CR, defined as CR + clinical CR [CRc, CR with residual skin abnormality not indicative of active disease]). Pts with CR/CRc undergoing allogeneic (allo)/autologous (auto) stem cell transplantation (SCT) discontinued TAG treatment; pts with CR/CRc not undergoing SCT and the remaining pts received TAG until disease progression/unacceptable toxicity. Long-term data were analyzed in all pts for safety and in 1L pts for efficacy.
Results
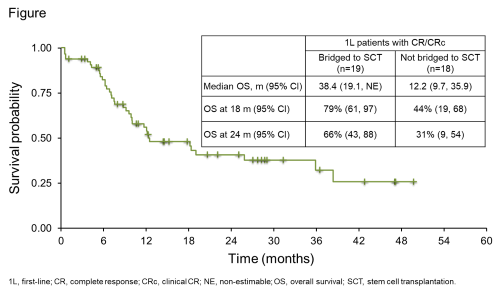
Of the 89 pts with BPDCN enrolled and treated (3 with TAG 7 mcg/kg and 86 with TAG 12 mcg/kg) over a 4-y period, 65 1L pts who received TAG monotherapy at a 12-mcg/kg dose were assessed for efficacy. The median age of 1L pts was 68 y (range: 22–84) and 80% were male. At study completion in March 2020 (cutoff date), the median follow-up was 34 m. Most common treatment-emergent adverse events (TEAEs; in >40% of pts [N=89]) were increased alanine (64%) or aspartate (60%) aminotransferase, hypoalbuminemia (51%), fatigue (44%), pyrexia (44%), thrombocytopenia (43%), nausea (42%), and peripheral edema (42%). Most TEAEs occurred during cycle 1; 19 (21%) pts had capillary leak syndrome (CLS; grade 2 in 12 [14%], grade 3 in 2 [2%], grade 4 in 2 [2%], grade 5 in 3 [3%]). All but 1 CLS events occurred in cycle 1; the median time (range) to CLS onset from therapy start was 6 d (3–51) and to CLS resolution was 5 d (2–69). CLS was managed by albumin supplementation, diuretics, and steroids, per recommended guidelines. For 1L pts, overall response rate was 75% (49/65) and 37/65 (57%) achieved CR/CRc, with a median time to CR/CRc of 39.0 d. Pts with CR/CRc received a median 4 TAG cycles (range: 1–76) and the median duration of CR/CRc was 24.9 m (95% CI: 3.8, not estimable; range: 1.0–57.4). Nineteen (51%) pts with CR/CRc bridged to SCT (13 allo, 6 auto) after a median time on study of 81 d (range: 27–182). The 18-m survival probability for 1L pts was 50% (95% CI: 36.7, 61.4) and median overall survival was 15.8 m (Figure).
Conclusion
In 1L pts with BPDCN, TAG monotherapy led to high response rates with durable responses. Overall, TAG therapy had a well-characterized and manageable safety profile. CLS management guidelines were developed and implemented during the trial. The high CR rate allowed pts to receive a median 4 cycles of TAG and 51% of responders were bridged to SCT. TAG is the first CD123-targeted therapy and first approved treatment for BPDCN in the US and EU. It is under investigation in other CD123-expressing hematologic malignancies.
Keyword(s): Hematological malignancy, Long-term follow-up, Targeted therapy
Abstract: EP427
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Clinical
Background
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematologic malignancy. CD123, the interleukin-3 (IL3) receptor alpha subunit, is overexpressed in BPDCN cells, making it an attractive therapeutic target. Tagraxofusp (TAG, SL-401) is a CD123-directed immunotherapy consisting of recombinant human IL3 and a truncated diphtheria toxin. TAG was FDA approved in 2018 for BPDCN treatment in adult and pediatric (≥2 y) patients (pts) and was recently approved in the EU as monotherapy for first line (1L) treatment in adults. Herein, we show long-term follow-up results from the largest prospective trial (NCT02113982) in pts with BPDCN.
Aims
To study the long-term safety and efficacy of TAG in pts with BPDCN.
Methods
This multicenter, open-label, phase 1/2 study consisted of Stages 1–4 (dose escalation, expansion, confirmatory, and continued access, respectively). Pts received TAG by IV infusion once daily on d 1–5 of a 21-d cycle; the dose was 7 or 12 mcg/kg in Stage 1 and 12 mcg/kg in Stages 2–4. The primary outcome was complete response (CR, defined as CR + clinical CR [CRc, CR with residual skin abnormality not indicative of active disease]). Pts with CR/CRc undergoing allogeneic (allo)/autologous (auto) stem cell transplantation (SCT) discontinued TAG treatment; pts with CR/CRc not undergoing SCT and the remaining pts received TAG until disease progression/unacceptable toxicity. Long-term data were analyzed in all pts for safety and in 1L pts for efficacy.
Results
Of the 89 pts with BPDCN enrolled and treated (3 with TAG 7 mcg/kg and 86 with TAG 12 mcg/kg) over a 4-y period, 65 1L pts who received TAG monotherapy at a 12-mcg/kg dose were assessed for efficacy. The median age of 1L pts was 68 y (range: 22–84) and 80% were male. At study completion in March 2020 (cutoff date), the median follow-up was 34 m. Most common treatment-emergent adverse events (TEAEs; in >40% of pts [N=89]) were increased alanine (64%) or aspartate (60%) aminotransferase, hypoalbuminemia (51%), fatigue (44%), pyrexia (44%), thrombocytopenia (43%), nausea (42%), and peripheral edema (42%). Most TEAEs occurred during cycle 1; 19 (21%) pts had capillary leak syndrome (CLS; grade 2 in 12 [14%], grade 3 in 2 [2%], grade 4 in 2 [2%], grade 5 in 3 [3%]). All but 1 CLS events occurred in cycle 1; the median time (range) to CLS onset from therapy start was 6 d (3–51) and to CLS resolution was 5 d (2–69). CLS was managed by albumin supplementation, diuretics, and steroids, per recommended guidelines. For 1L pts, overall response rate was 75% (49/65) and 37/65 (57%) achieved CR/CRc, with a median time to CR/CRc of 39.0 d. Pts with CR/CRc received a median 4 TAG cycles (range: 1–76) and the median duration of CR/CRc was 24.9 m (95% CI: 3.8, not estimable; range: 1.0–57.4). Nineteen (51%) pts with CR/CRc bridged to SCT (13 allo, 6 auto) after a median time on study of 81 d (range: 27–182). The 18-m survival probability for 1L pts was 50% (95% CI: 36.7, 61.4) and median overall survival was 15.8 m (Figure).

Conclusion
In 1L pts with BPDCN, TAG monotherapy led to high response rates with durable responses. Overall, TAG therapy had a well-characterized and manageable safety profile. CLS management guidelines were developed and implemented during the trial. The high CR rate allowed pts to receive a median 4 cycles of TAG and 51% of responders were bridged to SCT. TAG is the first CD123-targeted therapy and first approved treatment for BPDCN in the US and EU. It is under investigation in other CD123-expressing hematologic malignancies.
Keyword(s): Hematological malignancy, Long-term follow-up, Targeted therapy


