
Contributions
Abstract: EP420
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Central in the pathogenesis of APL, the PML/RARa fusion protein rewires metabolic pathways to feed anabolic cell growth. ATRA and ATO-based therapies render APL the most curable subtype of AML, yet approximately 5% of cases are resistant and 5 to 10% relapse.
Aims
To characterize the metabolic peculiarity and the energy requirements of PML-RARa expressing cells, to identify new therapeutic targets and strategies.
Methods
we analyzed primary samples from 5 APL with PMLRARa, normal CD34+ cells differentiated to promyelocytes and granulocyte, the U937-PR9 (PML-RARa inducible system), the NB4 APL cell line, and two NB4-ATO-resistant clones. We measured cell’s metabolism using a Seahorse Bioscience XFe96 analyzer, the rate of oxidation of each fuel was measured by means of mitochondrial respiration using the XF Mito Fuel Flex Test. We assessed expression of carnitine transporter CT2 (SLC22A16), PRDX, p-AMPK, MCL-1 and Glut 1 by western blot; SLC22A16 by q-RTPCR; cellular viability by ATP Glow Assay and ROS by mytosox assay.
Results
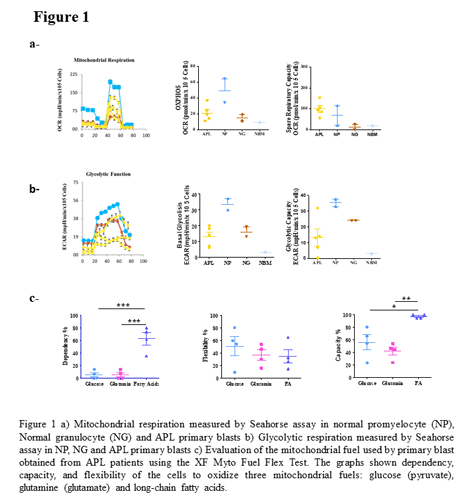
APL samples showed reduced respiratory and glycolytic capacity compared with normal promyelocytes (Fig. 1a). Expression of PML-RARa in U937-PR9 cell lines increases mitochondrial respiration rate (OXPHOS) and inhibits glycolysis. We evaluated dependency (cells’ reliance on a particular fuel source), flexibility (difference between fuel capacity and dependency, the oxidation of an alternative fuel) and capacity (the ability of a cell’s mitochondria to oxidize a fuel when other fuel pathways are inhibited). Primary APL blast cells depends mainly on fatty acids (FA), (FA: 63±20 % vs glucose: 5 ± 7 % and glutamine: 5 ± 7 %) (p<0.0005); they display a great flexibility, switching to glucose, glutamine or FAO to meet their energetic need (FA: 35±21 % vs glucose: 51± 30 % and glutamine: 36 ± 17 %) (Fig. 1b), consistent with the high adaptability of APL cells. Normal promyelocytes depend on FA and glucose for mitochondrial respiration, conversely APL blasts show enhanced FAO, increase in the carnitine transporter CT2, and lose pyruvate dependence, featuring flexibility for glucose utilization, and high lactate production when mitochondrial respiration is inhibited (Fig. 1c). The enhanced FAO and CT2 over-expression observed in APL blast was confirmed in the PR9 system after PML/RARa induction. ATO resistance involves a switch towards glycolysis, reduced viability at low glucose cc (p= 0.0008) and 1.5 times increase of the glucose transporter Glut1, increase in glycolytic and in mitochondrial ATP production; an increase in FAO confirmed by a higher sensitivity to Perexilin, inhibitor of CPTA1 (p=0.003), and a 1.7 fold increase in the expression of CT2. One clone shows also high level of ROS (p=0.0001), the cellular integrity is protected by increased levels of Periredoxin in both, and a 1.5 fold increase in pAMPK that induces FAO and glycolysis. The apoptosis escape in ATO resistant cells has been related to a concomitant increase (1.7 folds) in the expression of Mcl1.
Conclusion
FA is the principal fuel used by APL blasts and PML/RARa induces FAO. Metabolic shift from respiration to glycolysis and FAO in NB4-ATO resistant cells is mediated by pAMPK, the increase of glycolytic flux contributes to NADPH production and cell survival under oxidative stress. The increase in Mcl1 contributes to apoptotic resistance. ATO resistant patients could benefit from treatment with ATO in combination with glycolysis’ and FAO inhibitors.
Keyword(s): APL, Arsenic trioxide, ATRA resistant
Abstract: EP420
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Central in the pathogenesis of APL, the PML/RARa fusion protein rewires metabolic pathways to feed anabolic cell growth. ATRA and ATO-based therapies render APL the most curable subtype of AML, yet approximately 5% of cases are resistant and 5 to 10% relapse.
Aims
To characterize the metabolic peculiarity and the energy requirements of PML-RARa expressing cells, to identify new therapeutic targets and strategies.
Methods
we analyzed primary samples from 5 APL with PMLRARa, normal CD34+ cells differentiated to promyelocytes and granulocyte, the U937-PR9 (PML-RARa inducible system), the NB4 APL cell line, and two NB4-ATO-resistant clones. We measured cell’s metabolism using a Seahorse Bioscience XFe96 analyzer, the rate of oxidation of each fuel was measured by means of mitochondrial respiration using the XF Mito Fuel Flex Test. We assessed expression of carnitine transporter CT2 (SLC22A16), PRDX, p-AMPK, MCL-1 and Glut 1 by western blot; SLC22A16 by q-RTPCR; cellular viability by ATP Glow Assay and ROS by mytosox assay.
Results
APL samples showed reduced respiratory and glycolytic capacity compared with normal promyelocytes (Fig. 1a). Expression of PML-RARa in U937-PR9 cell lines increases mitochondrial respiration rate (OXPHOS) and inhibits glycolysis. We evaluated dependency (cells’ reliance on a particular fuel source), flexibility (difference between fuel capacity and dependency, the oxidation of an alternative fuel) and capacity (the ability of a cell’s mitochondria to oxidize a fuel when other fuel pathways are inhibited). Primary APL blast cells depends mainly on fatty acids (FA), (FA: 63±20 % vs glucose: 5 ± 7 % and glutamine: 5 ± 7 %) (p<0.0005); they display a great flexibility, switching to glucose, glutamine or FAO to meet their energetic need (FA: 35±21 % vs glucose: 51± 30 % and glutamine: 36 ± 17 %) (Fig. 1b), consistent with the high adaptability of APL cells. Normal promyelocytes depend on FA and glucose for mitochondrial respiration, conversely APL blasts show enhanced FAO, increase in the carnitine transporter CT2, and lose pyruvate dependence, featuring flexibility for glucose utilization, and high lactate production when mitochondrial respiration is inhibited (Fig. 1c). The enhanced FAO and CT2 over-expression observed in APL blast was confirmed in the PR9 system after PML/RARa induction. ATO resistance involves a switch towards glycolysis, reduced viability at low glucose cc (p= 0.0008) and 1.5 times increase of the glucose transporter Glut1, increase in glycolytic and in mitochondrial ATP production; an increase in FAO confirmed by a higher sensitivity to Perexilin, inhibitor of CPTA1 (p=0.003), and a 1.7 fold increase in the expression of CT2. One clone shows also high level of ROS (p=0.0001), the cellular integrity is protected by increased levels of Periredoxin in both, and a 1.5 fold increase in pAMPK that induces FAO and glycolysis. The apoptosis escape in ATO resistant cells has been related to a concomitant increase (1.7 folds) in the expression of Mcl1.

Conclusion
FA is the principal fuel used by APL blasts and PML/RARa induces FAO. Metabolic shift from respiration to glycolysis and FAO in NB4-ATO resistant cells is mediated by pAMPK, the increase of glycolytic flux contributes to NADPH production and cell survival under oxidative stress. The increase in Mcl1 contributes to apoptotic resistance. ATO resistant patients could benefit from treatment with ATO in combination with glycolysis’ and FAO inhibitors.
Keyword(s): APL, Arsenic trioxide, ATRA resistant


