
Contributions
Abstract: EP417
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Despite emerging insight into the molecular pathogenesis of acute myeloid leukaemia (AML), few new therapeutic drugs have been approved and overall survival in elderly patients remains poor. Tumor-stromal interactions in AML are important for disease development and therapy resistance, and bone marrow stroma seem like an attractive therapeutic target. Inhibition of colony stimulating factor 1 receptor (CSF1R) is proposed to be an effective target to block infiltrating monocytes and tumor-associated macrophages that support tumor growth. Our laboratory is currently developing a novel class of small molecule kinase inhibitors towards FLT3 and CSF1R through a collaboration with BCI Pharma to target high-risk mutations. We propose an increased beneficial effect of conventional and novel therapeutics by targeting the supportive microenvironment in addition to direct leukemic cell death. Experimental evidence positioning inhibitors of CSF1R as treatment should, together with data from preclinical and early phase clinical trials, facilitate translation and clinical development of CSF1R-targeted therapy in AML.
Aims
We propose the potential of CSF1R/FLT3-targeted therapy as well as to elucidate the supportive mechanisms of the tumor microenvironment in AML.
Methods
We have established IC-50 values of various CSF1R and FLT3 inhibitors on multiple leukemic and stromal cell lines by high-throughput dose-response WST-1 assay. To further investigate the interactions of leukemic cells with the stromal compartment of the bone marrow in AML, a 3D co-culture model using alginate gel was established. The leukemic cell line MV-4-11, and the stromal cell line HS-5 were incapsulated in different ratios in 5% alginate gel and exposed to a few selected CSF1R and FLT3 inhibitors, as well as stromal-conditioned media. An in vivo maximum tolerable dose (MTD) experiment was performed for the selected FLT3 and CSF1R inhibitors. We are currently running an orthotopic MV-4-11 experiment were FLT3 and CSF1R inhibitors are given in sequence and as monotherapies. Malignancy and effect of treatment are assessed through bioluminescent imaging.
Results
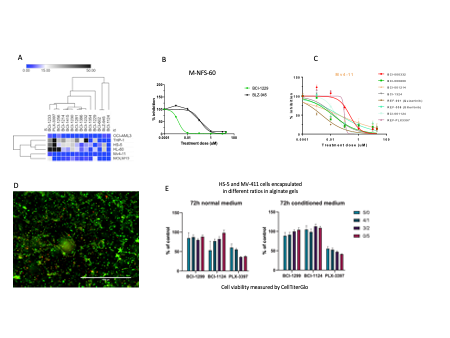
Dose-response assays of several CSF1R and FLT3 inhibitors on leukemic and stromal cell lines showed significant inhibition and reduced viability by several compounds (Figure 1A),where the most potent inhibitors were selected for further in vitro and in vivo experiments. CSF1R inhibitor BCI-1229 is more potent than the commercially available inhibitor BLZ-945 in the CSF1R-dependent cell line M-NFS-60 (Figure 1B). Several FLT3 inhibitors show inhibitory effect on the cell line MV-4-11 that is comparable to commercially available reference compounds (Figure 1C). Further, the co-culture alginate gel is compatible withthe leukemic and stromal cells illustrated by live/dead imaging (Figure 1D). Interestingly, FLT3-inhibitors has significantly reduced effect on leukemic and stromal cells cultured in stromal-conditioned media after 72 hours (Figure 1E).
Conclusion
In summary, we employ a novel approach to develop combined CSF1R/FLT3 targeted therapy for AML to assess the protective tumor microenvironment and by direct leukemic cell death. Ultimately, our goal is to facilitate translational medicine by performing rigorous preclinical experiments and to select compounds to be tested in a clinical trial setting combined with conventional and other novel therapeutics.
Keyword(s): Bone marrow stroma, Flt3 inhibitor, Tyrosine kinase inhibitor
Abstract: EP417
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Despite emerging insight into the molecular pathogenesis of acute myeloid leukaemia (AML), few new therapeutic drugs have been approved and overall survival in elderly patients remains poor. Tumor-stromal interactions in AML are important for disease development and therapy resistance, and bone marrow stroma seem like an attractive therapeutic target. Inhibition of colony stimulating factor 1 receptor (CSF1R) is proposed to be an effective target to block infiltrating monocytes and tumor-associated macrophages that support tumor growth. Our laboratory is currently developing a novel class of small molecule kinase inhibitors towards FLT3 and CSF1R through a collaboration with BCI Pharma to target high-risk mutations. We propose an increased beneficial effect of conventional and novel therapeutics by targeting the supportive microenvironment in addition to direct leukemic cell death. Experimental evidence positioning inhibitors of CSF1R as treatment should, together with data from preclinical and early phase clinical trials, facilitate translation and clinical development of CSF1R-targeted therapy in AML.
Aims
We propose the potential of CSF1R/FLT3-targeted therapy as well as to elucidate the supportive mechanisms of the tumor microenvironment in AML.
Methods
We have established IC-50 values of various CSF1R and FLT3 inhibitors on multiple leukemic and stromal cell lines by high-throughput dose-response WST-1 assay. To further investigate the interactions of leukemic cells with the stromal compartment of the bone marrow in AML, a 3D co-culture model using alginate gel was established. The leukemic cell line MV-4-11, and the stromal cell line HS-5 were incapsulated in different ratios in 5% alginate gel and exposed to a few selected CSF1R and FLT3 inhibitors, as well as stromal-conditioned media. An in vivo maximum tolerable dose (MTD) experiment was performed for the selected FLT3 and CSF1R inhibitors. We are currently running an orthotopic MV-4-11 experiment were FLT3 and CSF1R inhibitors are given in sequence and as monotherapies. Malignancy and effect of treatment are assessed through bioluminescent imaging.
Results
Dose-response assays of several CSF1R and FLT3 inhibitors on leukemic and stromal cell lines showed significant inhibition and reduced viability by several compounds (Figure 1A),where the most potent inhibitors were selected for further in vitro and in vivo experiments. CSF1R inhibitor BCI-1229 is more potent than the commercially available inhibitor BLZ-945 in the CSF1R-dependent cell line M-NFS-60 (Figure 1B). Several FLT3 inhibitors show inhibitory effect on the cell line MV-4-11 that is comparable to commercially available reference compounds (Figure 1C). Further, the co-culture alginate gel is compatible withthe leukemic and stromal cells illustrated by live/dead imaging (Figure 1D). Interestingly, FLT3-inhibitors has significantly reduced effect on leukemic and stromal cells cultured in stromal-conditioned media after 72 hours (Figure 1E).

Conclusion
In summary, we employ a novel approach to develop combined CSF1R/FLT3 targeted therapy for AML to assess the protective tumor microenvironment and by direct leukemic cell death. Ultimately, our goal is to facilitate translational medicine by performing rigorous preclinical experiments and to select compounds to be tested in a clinical trial setting combined with conventional and other novel therapeutics.
Keyword(s): Bone marrow stroma, Flt3 inhibitor, Tyrosine kinase inhibitor


