
Contributions
Abstract: EP412
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
In recent years, several targeted therapies have received the approval for treating AML. Nevertheless, for many patients 7+3 chemotherapy regimens remain as the treatment of choice, and so, their lack of efficacy are the main cause of death in the disease. Therefore, there is still a necessity of more appropriate and personalized treatments, which try to avoid resistance development.
Aims
In this study, we elucidate the implication of splicing factor SR proteins in cytarabine resistance and propose new combinational therapies.
Methods
The public database GEPIA was used to compare the expression levels of genes codifying SR proteins from the LAML (TGCA) repository versus the GTEx repository (controls). Expression levels of SRRM2 and SRSF12 in controls, AML patients, and other myeloid disorders (MPNs and MDS) were studied by qPCR (n=54). The analysis of the proteomic profile associated to cytarabine treatment resistance was performed by LC-MSMS after IMAC enrichment (n=3). Expression levels of SR proteins and phospho-SR proteins were assayed by immunohistochemistry of paired bone marrow smears from 7 AML patients, and at the diagnosis moment of different cytarabine-response groups (n=64). The efficacy of different spliceosome inhibitors (H3B-8800, Madrasin, SPHINX31, and SRPKIN-1) and their combination with cytarabine were studied in AML cell lines.
Results
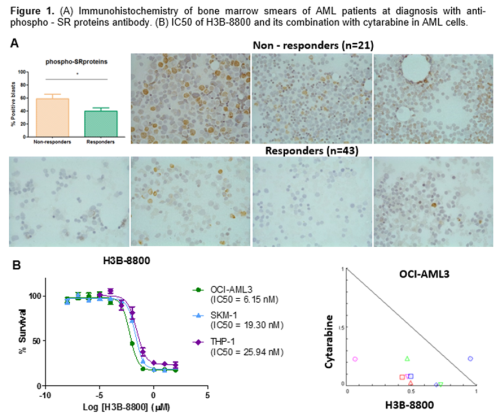
Public data showed differences in the expression levels of 4 SR proteins (SRRM2, SRSF12, SRSF9, and U2AF1L5). SRRM2 and SRSF12 genes exhibited overexpression in bone marrow of AML patients compared to controls, MPNs and MDS. Likewise, proteomic analysis identified an increase in the phosphorylation of SRRM2, among other SR proteins comparing paired samples before and after cytarabine resistance development. These data were validated by immunohistochemistry in which the levels of phospho-SR proteins were significantly lower in cytarabine-responders AML patients compared to non-responders (figure 1A). Finally, different spliceosome inhibitors demonstrated in vitro efficacy in the nanomolar (H3B-8800) or micromolar range (Madrasin, SPHINX31, and SRPKIN-1). The combination of cytarabine plus H3B-8800 showed synergism activity in OCI-AML3 cells (figure 1B).
Conclusion
The spliceosome pathway seems to be involved in cytarabine resistance in AML patients. The levels of SRRM2 and SRSF12 are higher in AML compared to controls and other myeloid disorders. The levels of phospho-SR proteins increase after cytarabine treatment in AML patients that relapse, and are especially higher at diagnosis in refractory patients. Certainly, the study of phospho-SR proteins at diagnosis could serve as a possible predictive biomarker of cytarabine response. The combination of cytarabine plus spliceosome inhibitors as H3B-8800, a small molecule that binds to the SF3b spliceosome complex, could improve the response in AML patients.
Keyword(s): Acute myeloid leukemia, Chemotherapy, Cytarabine, Resistance
Abstract: EP412
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
In recent years, several targeted therapies have received the approval for treating AML. Nevertheless, for many patients 7+3 chemotherapy regimens remain as the treatment of choice, and so, their lack of efficacy are the main cause of death in the disease. Therefore, there is still a necessity of more appropriate and personalized treatments, which try to avoid resistance development.
Aims
In this study, we elucidate the implication of splicing factor SR proteins in cytarabine resistance and propose new combinational therapies.
Methods
The public database GEPIA was used to compare the expression levels of genes codifying SR proteins from the LAML (TGCA) repository versus the GTEx repository (controls). Expression levels of SRRM2 and SRSF12 in controls, AML patients, and other myeloid disorders (MPNs and MDS) were studied by qPCR (n=54). The analysis of the proteomic profile associated to cytarabine treatment resistance was performed by LC-MSMS after IMAC enrichment (n=3). Expression levels of SR proteins and phospho-SR proteins were assayed by immunohistochemistry of paired bone marrow smears from 7 AML patients, and at the diagnosis moment of different cytarabine-response groups (n=64). The efficacy of different spliceosome inhibitors (H3B-8800, Madrasin, SPHINX31, and SRPKIN-1) and their combination with cytarabine were studied in AML cell lines.
Results
Public data showed differences in the expression levels of 4 SR proteins (SRRM2, SRSF12, SRSF9, and U2AF1L5). SRRM2 and SRSF12 genes exhibited overexpression in bone marrow of AML patients compared to controls, MPNs and MDS. Likewise, proteomic analysis identified an increase in the phosphorylation of SRRM2, among other SR proteins comparing paired samples before and after cytarabine resistance development. These data were validated by immunohistochemistry in which the levels of phospho-SR proteins were significantly lower in cytarabine-responders AML patients compared to non-responders (figure 1A). Finally, different spliceosome inhibitors demonstrated in vitro efficacy in the nanomolar (H3B-8800) or micromolar range (Madrasin, SPHINX31, and SRPKIN-1). The combination of cytarabine plus H3B-8800 showed synergism activity in OCI-AML3 cells (figure 1B).

Conclusion
The spliceosome pathway seems to be involved in cytarabine resistance in AML patients. The levels of SRRM2 and SRSF12 are higher in AML compared to controls and other myeloid disorders. The levels of phospho-SR proteins increase after cytarabine treatment in AML patients that relapse, and are especially higher at diagnosis in refractory patients. Certainly, the study of phospho-SR proteins at diagnosis could serve as a possible predictive biomarker of cytarabine response. The combination of cytarabine plus spliceosome inhibitors as H3B-8800, a small molecule that binds to the SF3b spliceosome complex, could improve the response in AML patients.
Keyword(s): Acute myeloid leukemia, Chemotherapy, Cytarabine, Resistance


