
Contributions
Abstract: EP408
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
The prognostic classification of acute myeloid leukemia (AML) proposed by the European Leukemia Net (2017) is based on the presence of mutations in FLT3 (ITD), NPM1, CEBPA, RUNX1, ASXL1 and TP53. However, there is little information about the role of mutational load in the genes involved in AML. The impact of mutant ASXL1, DNMT3A, JAK2, TET2, TP53, and NPM1 variant allelic frequency (VAF) in newly diagnosed acute myeloid leukemia has been previously described (Short NJ, Blood advances 2020; Sasaki K, Cancer 2020), but not in other genes involved in AML.
Aims
The objective of the current study was to evaluate the prognostic impact of the VAF, detected by NGS in a custom panel of genes associated to myeloid pathology in a cohort of AML patients, who have been treated in the healthcare practice at the Hospital 12 de Octubre in Madrid, according to the type of treatment received (intensive treatment, hypomethylating agents or low-dose cytarabine).
Methods
The study was conducted in a series of 535 patients diagnosed with AML, whose median age is 67 years. Patients evaluated for Overall Survival (OS) and Relapse-Free Survival (RFS) were 497 cases: 238 cases had received intensive treatment (3+7 schemes or similar), 113 had received hypomethylating agents (azacitidine or decitabine) and 146 with low-dose cytarabine schemes. 38 patients received supportive care and were excluded from the analyses. The mutational profile at diagnosis was identified by NGS (Ion Torrent System), using a panel of 41 genes involved in myeloid pathologies: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, DNMT3A, EPAS1, EPOR, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KDM6A, KIT, KMT2A, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PRPF40B, RAD21, RUNX1, SETBP1, SF3A1, SF3B1, SH2B3, SMC1A, SRSF2, STAG2, TET2, THPO, TP53, U2AF1, VHL, WT1 and ZRSR2.
Pathogenic or likely pathogenic variants with >1% VAF were considered. Mutational load (VAF) was assessed as a continuous variable.
Results
The percentage of mutations in older and younger patients was 97.7% and 89.8% respectively.
Younger AML patients have more frequently mutated NPM1 (34.4% vs 18.6%; P=0.004) and patients over 60 years old have more frequently mutated RUNX1 (18.4% vs 8.6%; p=0.005) and ASXL1 ((16.6% vs 3.9%; p<0.001) genes.
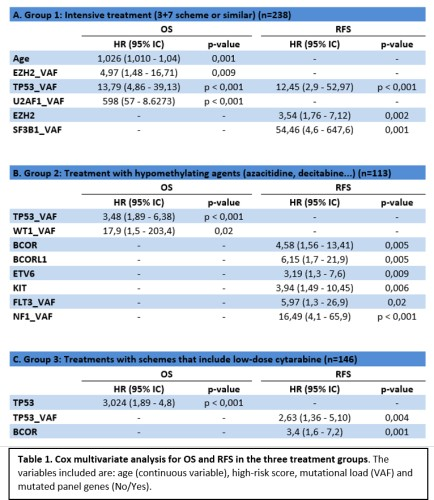
In the Cox multivariate analysis, the variables associated with worse OS in patients with intensive treatment were older age or higher TP53_VAF, U2AF1_VAF and EZH2_VAF; and higher TP53_VAF or the presence of EZH2-mutated were found as adverse factors for RFS. In patients who received hypomethylating agents, the variables associated with lower OS were higher TP53_VAF and WT1_VAF; and the presence of mutations in the BCOR, KIT or ETV6 genes was associated to worse RFS. Finally, in patients treated with low-dose cytarabine, TP53 is associated with worse OS and a higher mutational load of TP53 or mutations in the BCOR gene are associated with a worse RFS.
Conclusion
The impact of TP53 mutational load has prognostic influence on the 3 treatment groups. There are other genes where the impact of their mutational load depends on the treatment received: Variants-VAF of splicing genes such as U2AF1 and SF3B1 and chromatin modifying genes such as EZH2 only has an influence on the intensive treatment group.
Keyword(s): Acute myeloid leukemia, Mutation analysis
Abstract: EP408
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
The prognostic classification of acute myeloid leukemia (AML) proposed by the European Leukemia Net (2017) is based on the presence of mutations in FLT3 (ITD), NPM1, CEBPA, RUNX1, ASXL1 and TP53. However, there is little information about the role of mutational load in the genes involved in AML. The impact of mutant ASXL1, DNMT3A, JAK2, TET2, TP53, and NPM1 variant allelic frequency (VAF) in newly diagnosed acute myeloid leukemia has been previously described (Short NJ, Blood advances 2020; Sasaki K, Cancer 2020), but not in other genes involved in AML.
Aims
The objective of the current study was to evaluate the prognostic impact of the VAF, detected by NGS in a custom panel of genes associated to myeloid pathology in a cohort of AML patients, who have been treated in the healthcare practice at the Hospital 12 de Octubre in Madrid, according to the type of treatment received (intensive treatment, hypomethylating agents or low-dose cytarabine).
Methods
The study was conducted in a series of 535 patients diagnosed with AML, whose median age is 67 years. Patients evaluated for Overall Survival (OS) and Relapse-Free Survival (RFS) were 497 cases: 238 cases had received intensive treatment (3+7 schemes or similar), 113 had received hypomethylating agents (azacitidine or decitabine) and 146 with low-dose cytarabine schemes. 38 patients received supportive care and were excluded from the analyses. The mutational profile at diagnosis was identified by NGS (Ion Torrent System), using a panel of 41 genes involved in myeloid pathologies: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, DNMT3A, EPAS1, EPOR, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KDM6A, KIT, KMT2A, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PRPF40B, RAD21, RUNX1, SETBP1, SF3A1, SF3B1, SH2B3, SMC1A, SRSF2, STAG2, TET2, THPO, TP53, U2AF1, VHL, WT1 and ZRSR2.
Pathogenic or likely pathogenic variants with >1% VAF were considered. Mutational load (VAF) was assessed as a continuous variable.
Results
The percentage of mutations in older and younger patients was 97.7% and 89.8% respectively.
Younger AML patients have more frequently mutated NPM1 (34.4% vs 18.6%; P=0.004) and patients over 60 years old have more frequently mutated RUNX1 (18.4% vs 8.6%; p=0.005) and ASXL1 ((16.6% vs 3.9%; p<0.001) genes.
In the Cox multivariate analysis, the variables associated with worse OS in patients with intensive treatment were older age or higher TP53_VAF, U2AF1_VAF and EZH2_VAF; and higher TP53_VAF or the presence of EZH2-mutated were found as adverse factors for RFS. In patients who received hypomethylating agents, the variables associated with lower OS were higher TP53_VAF and WT1_VAF; and the presence of mutations in the BCOR, KIT or ETV6 genes was associated to worse RFS. Finally, in patients treated with low-dose cytarabine, TP53 is associated with worse OS and a higher mutational load of TP53 or mutations in the BCOR gene are associated with a worse RFS.

Conclusion
The impact of TP53 mutational load has prognostic influence on the 3 treatment groups. There are other genes where the impact of their mutational load depends on the treatment received: Variants-VAF of splicing genes such as U2AF1 and SF3B1 and chromatin modifying genes such as EZH2 only has an influence on the intensive treatment group.
Keyword(s): Acute myeloid leukemia, Mutation analysis


