
Contributions
Abstract: EP393
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Canonical WNT signaling is a critical pathway for normal development. β-catenin, the central mediator, is constitutively degraded in the absence of an agonist. Following activation, stabilized β-catenin localizes to the nucleus and associates with TCF/LEF factors to create a transcription complex that targets genes involved in survival, proliferation, cell fate determination and stemness. WNT signaling has been identified as one of the most frequently dysregulated pathways in acute myeloid leukemia (AML), with β-catenin prominently overexpressed at mRNA and protein levels in patients where it is associated with poor clinical outcomes. In addition, overexpression of β-catenin in human hematopoietic stem cells (HSC) blocks multilineage differentiation, mimicking the AML phenotype. However, whether canonical WNT signaling has an essential role in AML remains contentious due to leakiness in gene targeting and RNAi approaches and failure to exclude possible redundancy in respect of its close homologue, γ-catenin. Furthermore, only a subset of patients and cell lines demonstrate nuclear translocation of β-catenin, suggesting dependency is unlikely to be universal in AML.
Aims
To establish whether AML cells are dependent on β-catenin and/or γ-catenin expression for their growth and survival using CRISPR-Cas9 to genetically edit cells.
Methods
We employed CRISPR-Cas9 lentivirus to generate single and dual knockout of β-catenin (βKO) and γ-catenin (γKO) to study the functional impact of complete knockout of canonical WNT signalling in AML cell lines. All KO cultures were validated by sequencing alongside ablation of protein expression and TCF reporter response. Equivalent non-targeted CRISPR-Cas9 lines were used as controls.
Results
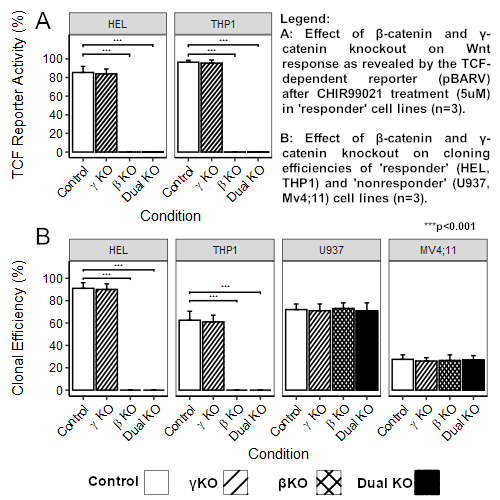
As previously shown, we found that AML cell lines are heterogeneous in their response to β-catenin agonists with “responders” (such as HEL and THP1) showing strong nuclear localization of β-catenin and TCF-dependent transcriptional responses to agonist, whilst “non-responders” (such as U937 and Mv4;11) failed to localize β-catenin to the nucleus and generated no transcriptional response. We generated complete knockouts of β-catenin and γ-catenin (singly and in combination) in all these lines starting with clonal populations co-expressing a TCF-dependent YFP reporter. βKO resulted in ablation of TCF reporter signal in responder lines, whilst γKO had no effect (Fig.1A), showing that β-catenin is essential for canonical WNT signaling and its role was not redundant in respect of γ-catenin. We found βKO was incompatible with clonal growth in responder lines whereas γKO had no effect. Predictably, colony forming efficiency of non-responder lines was unaffected by any KO condition (Fig.1B). Clonal growth of βKO lines could be rescued by co-cultivation with WT cells (but not with WT conditioned medium, supplementary cytokines, or stromal support) suggesting rescue by a short-range/labile autocrine factor or engagement with a cell surface molecule. Rescued clonal βKO cultures grew autonomously in bulk culture when purified by FACS, but could not be re-cloned, demonstrating stable and continuing dependence on β-catenin for clonal growth.
Conclusion
Together these data show that β-catenin has functional relevance for clonal growth in WNT-responsive AML lines but not in lines that are unresponsive to WNT agonist. We saw no dependency on γ-catenin in any context. We are currently performing RNAseq to identify candidate genes mediating β-catenin-dependent survival under clonal conditions.
Keyword(s): Acute myeloid leukemia, Beta-catenin, Clonal expansion, Wnt
Abstract: EP393
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Canonical WNT signaling is a critical pathway for normal development. β-catenin, the central mediator, is constitutively degraded in the absence of an agonist. Following activation, stabilized β-catenin localizes to the nucleus and associates with TCF/LEF factors to create a transcription complex that targets genes involved in survival, proliferation, cell fate determination and stemness. WNT signaling has been identified as one of the most frequently dysregulated pathways in acute myeloid leukemia (AML), with β-catenin prominently overexpressed at mRNA and protein levels in patients where it is associated with poor clinical outcomes. In addition, overexpression of β-catenin in human hematopoietic stem cells (HSC) blocks multilineage differentiation, mimicking the AML phenotype. However, whether canonical WNT signaling has an essential role in AML remains contentious due to leakiness in gene targeting and RNAi approaches and failure to exclude possible redundancy in respect of its close homologue, γ-catenin. Furthermore, only a subset of patients and cell lines demonstrate nuclear translocation of β-catenin, suggesting dependency is unlikely to be universal in AML.
Aims
To establish whether AML cells are dependent on β-catenin and/or γ-catenin expression for their growth and survival using CRISPR-Cas9 to genetically edit cells.
Methods
We employed CRISPR-Cas9 lentivirus to generate single and dual knockout of β-catenin (βKO) and γ-catenin (γKO) to study the functional impact of complete knockout of canonical WNT signalling in AML cell lines. All KO cultures were validated by sequencing alongside ablation of protein expression and TCF reporter response. Equivalent non-targeted CRISPR-Cas9 lines were used as controls.
Results
As previously shown, we found that AML cell lines are heterogeneous in their response to β-catenin agonists with “responders” (such as HEL and THP1) showing strong nuclear localization of β-catenin and TCF-dependent transcriptional responses to agonist, whilst “non-responders” (such as U937 and Mv4;11) failed to localize β-catenin to the nucleus and generated no transcriptional response. We generated complete knockouts of β-catenin and γ-catenin (singly and in combination) in all these lines starting with clonal populations co-expressing a TCF-dependent YFP reporter. βKO resulted in ablation of TCF reporter signal in responder lines, whilst γKO had no effect (Fig.1A), showing that β-catenin is essential for canonical WNT signaling and its role was not redundant in respect of γ-catenin. We found βKO was incompatible with clonal growth in responder lines whereas γKO had no effect. Predictably, colony forming efficiency of non-responder lines was unaffected by any KO condition (Fig.1B). Clonal growth of βKO lines could be rescued by co-cultivation with WT cells (but not with WT conditioned medium, supplementary cytokines, or stromal support) suggesting rescue by a short-range/labile autocrine factor or engagement with a cell surface molecule. Rescued clonal βKO cultures grew autonomously in bulk culture when purified by FACS, but could not be re-cloned, demonstrating stable and continuing dependence on β-catenin for clonal growth.

Conclusion
Together these data show that β-catenin has functional relevance for clonal growth in WNT-responsive AML lines but not in lines that are unresponsive to WNT agonist. We saw no dependency on γ-catenin in any context. We are currently performing RNAseq to identify candidate genes mediating β-catenin-dependent survival under clonal conditions.
Keyword(s): Acute myeloid leukemia, Beta-catenin, Clonal expansion, Wnt


