
Contributions
Abstract: EP383
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
About 25% of patients with acute myeloid leukemia (AML) have internal tandem duplications (ITDs) in the Fms-Like Tyrosine kinase-3 (FLT3) receptor, leading to constitutive activation of the receptor and downstream signaling pathways, and uncontrolled cell division. Several approved and investigational small molecule kinase inhibitors are available, although all kinase inhibitors show off target effects.
Aims
We investigated a novel library of highly selective FLT3-targeting inhibitors built on the chemical backbone of the anti-HIV drug tenofovir, aiming to identify new clinically safe inhibitors with superior FLT3-specificity and reduced off-target effects.
Methods
A panel of 40 analogs with kinase inhibitory properties were synthetized and screened in multiple cell lines expressing wild type (wt) and ITD-mutated FLT3 receptor using the WST-1 assay to define anti-leukemic effect IC50, validated by Hoechst. The novel inhibitor BCI 332 was selected for further evaluation, using quizartinib as a reference compound. BCI 332 was investigated for in vivo toxicity and efficacy in an NSG (NOD/SCID IL2rγnull) subcutaneous mouse model using MV4-11 (ITD/null) cells. Functional assessments of anti-leukemic effects (Annexin V/PI and cell cycle analysis) were performed by flow cytometry in MOLM-13 (FLT3 ITD/wt) and OCI-AML3 (FLT3 wt/wt) cells. Downstream effects of FLT3-inhibition were assessed by mass cytometry (CyTOF) in ex vivo treated mononuclear cells (bone marrow) from healthy donors and FLT3 wt (n=3) and FLT3-ITD mutated (n=3) AML patients, in addition to the cell lines MOLM-13 and OCI-AML3. Samples were stained with a panel of 19 extracellular and 13 intracellular antibodies, and cell subsets were identified by unsupervised clustering based on surface marker expression using the phenograph algorithm.
Results
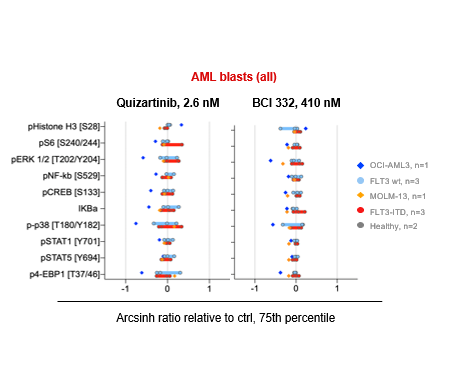
We identified several potent inhibitors with differential effect on cells expressing FLT3 wt and FLT3-ITD. The inhibitor BCI 332 showed selectivity towards FLT3-ITD, and was therefore further assessed. In vivo toxicity and efficacy studies demonstrated low toxicity and potent anti-leukemic effect. Functional assays of MOLM-13 and OCI-AML3 cells showed that BCI-332 induced apoptosis in FLT3-ITD cells, although less potent than quizartinib. CyTOF analyses of healthy (monocytes and CD4 T cells) and leukemic cell subsets revealed heterogeneous response profiles in patient samples, although we did not identify significant differences between FLT3 wt and -ITD mutated samples (Figure).
Conclusion
The novel inhibitor BCI 332 induced apoptosis in FLT3-ITD mutated cells, and our results indicate higher specificity towards FLT3-ITD than the reference compound quizartinib. Our analyses did not reveal an ITD-specific intracellular response profile to BC I332, likely due to a small sample size and high inter-patient heterogeneity. Thus, work continues to expand the patient cohort, and to provide assessment of inhibition in immunophenotypically distinct leukemic cell subsets.
Keyword(s): AML, Apoptosis, Flt3-ITD, Tyrosine kinase inhibitor
Abstract: EP383
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
About 25% of patients with acute myeloid leukemia (AML) have internal tandem duplications (ITDs) in the Fms-Like Tyrosine kinase-3 (FLT3) receptor, leading to constitutive activation of the receptor and downstream signaling pathways, and uncontrolled cell division. Several approved and investigational small molecule kinase inhibitors are available, although all kinase inhibitors show off target effects.
Aims
We investigated a novel library of highly selective FLT3-targeting inhibitors built on the chemical backbone of the anti-HIV drug tenofovir, aiming to identify new clinically safe inhibitors with superior FLT3-specificity and reduced off-target effects.
Methods
A panel of 40 analogs with kinase inhibitory properties were synthetized and screened in multiple cell lines expressing wild type (wt) and ITD-mutated FLT3 receptor using the WST-1 assay to define anti-leukemic effect IC50, validated by Hoechst. The novel inhibitor BCI 332 was selected for further evaluation, using quizartinib as a reference compound. BCI 332 was investigated for in vivo toxicity and efficacy in an NSG (NOD/SCID IL2rγnull) subcutaneous mouse model using MV4-11 (ITD/null) cells. Functional assessments of anti-leukemic effects (Annexin V/PI and cell cycle analysis) were performed by flow cytometry in MOLM-13 (FLT3 ITD/wt) and OCI-AML3 (FLT3 wt/wt) cells. Downstream effects of FLT3-inhibition were assessed by mass cytometry (CyTOF) in ex vivo treated mononuclear cells (bone marrow) from healthy donors and FLT3 wt (n=3) and FLT3-ITD mutated (n=3) AML patients, in addition to the cell lines MOLM-13 and OCI-AML3. Samples were stained with a panel of 19 extracellular and 13 intracellular antibodies, and cell subsets were identified by unsupervised clustering based on surface marker expression using the phenograph algorithm.
Results
We identified several potent inhibitors with differential effect on cells expressing FLT3 wt and FLT3-ITD. The inhibitor BCI 332 showed selectivity towards FLT3-ITD, and was therefore further assessed. In vivo toxicity and efficacy studies demonstrated low toxicity and potent anti-leukemic effect. Functional assays of MOLM-13 and OCI-AML3 cells showed that BCI-332 induced apoptosis in FLT3-ITD cells, although less potent than quizartinib. CyTOF analyses of healthy (monocytes and CD4 T cells) and leukemic cell subsets revealed heterogeneous response profiles in patient samples, although we did not identify significant differences between FLT3 wt and -ITD mutated samples (Figure).

Conclusion
The novel inhibitor BCI 332 induced apoptosis in FLT3-ITD mutated cells, and our results indicate higher specificity towards FLT3-ITD than the reference compound quizartinib. Our analyses did not reveal an ITD-specific intracellular response profile to BC I332, likely due to a small sample size and high inter-patient heterogeneity. Thus, work continues to expand the patient cohort, and to provide assessment of inhibition in immunophenotypically distinct leukemic cell subsets.
Keyword(s): AML, Apoptosis, Flt3-ITD, Tyrosine kinase inhibitor


