
Contributions
Abstract: EP377
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
The incidence of acute myeloid leukemia (AML) increases with age and elderly patients display increased frequency of unfavorable cytogenetics. However, when compared to similarly-treated younger patients from the same cytogenetic risk group, elderly patients still experience lower overall survival, indicating that age-related differences extrinsic to hematopoietic cells contribute to AML therapy resistance and disease relapse. Interactions between leukemic cells and the bone marrow (BM) microenvironment are crucial for myeloid leukemia progression. Indeed, leukemic cells have the ability to shape their microenvironment in order to support their expansion and BM stromal cells can support malignant hematopoiesis at the expense of normal hematopoiesis. The BM microenvironment also changes during ageing, contributing to the age-associated decline in adaptive immunity, and platelet-myeloid bias of cellular output. These changes can potentially contribute to the increased prevalence of myeloid malignancies seen in the elderly population.
Aims
Address the potential role of the aged microenvironment in myeloid leukemogenesis by investigating the correlation between AML- and ageing-related changes in the BM microenvironment.
Methods
We developed a murine model of Cebpa mutant AML, where Cebpa mutant leukemic initiating cells (LICs) were injected into sub-lethally irradiated young mice, reproducibly leading to AML in 4 weeks. Flow cytometry was used to study the changes that occur to the abundance of BM niche during AML development. Molecular profiling of leukemic cells and BM stromal cells during leukemogenesis was performed, and the identified changes were overlaid with those occurring during physiological ageing.
Results
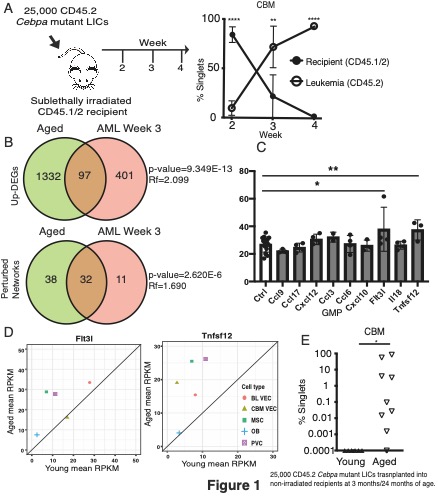
In our Cebpa mutant AML model, the 2-, 3- and 4-week time points represented 3 key stages of leukemia progression: i) an early expansion phase with no overt leukemia; ii) a late expansion phase with leukemia cells highly abundant in hematopoietic organs, and iii) a terminal phase where normal BM hematopoiesis is displaced (Figure 1A). As most AML cells occupy the central BM (CBM), we focused on CBM vascular endothelial cells (VECs) and perivascular cells (PVCs). We observed a decrease in the number of CBM VECs in leukemic, compared to mock transplanted mice, which was similar to that observed during normal ageing. CBM VECs and PVCs were profiled by RNA sequencing at the 3 key time points after LIC transplantation, with cells from mock transplanted mice profiled as controls. In CBM VECs, we observed a significant overlap between the genes and process networks altered during ageing and those altered at week 3 of AML (Figure 1B). In addition, several cytokines produced by leukemic cells were capable of expanding myeloid progenitors (the closest normal counterpart of LIC) in vivo. These cytokines were also upregulated in aged BM stromal cells, consistent with the aged BM microenvironment sharing characteristics with that adapted to AML (Figure 1C-D). This hypothesis was confirmed when we observed leukemia engraftment only in non-irradiated aged mice injected with Cebpa mutant LICs but not in young mice (Figure 1E).
Conclusion
Molecular and functional similarities exist between the BM microenvironment generated during leukemia progression and physiological ageing. These similarities may contribute to increased AML incidence and progression by providing support for the expansion of myeloid progenitors, including LICs.
Keyword(s): AML, Microenvironment
Abstract: EP377
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
The incidence of acute myeloid leukemia (AML) increases with age and elderly patients display increased frequency of unfavorable cytogenetics. However, when compared to similarly-treated younger patients from the same cytogenetic risk group, elderly patients still experience lower overall survival, indicating that age-related differences extrinsic to hematopoietic cells contribute to AML therapy resistance and disease relapse. Interactions between leukemic cells and the bone marrow (BM) microenvironment are crucial for myeloid leukemia progression. Indeed, leukemic cells have the ability to shape their microenvironment in order to support their expansion and BM stromal cells can support malignant hematopoiesis at the expense of normal hematopoiesis. The BM microenvironment also changes during ageing, contributing to the age-associated decline in adaptive immunity, and platelet-myeloid bias of cellular output. These changes can potentially contribute to the increased prevalence of myeloid malignancies seen in the elderly population.
Aims
Address the potential role of the aged microenvironment in myeloid leukemogenesis by investigating the correlation between AML- and ageing-related changes in the BM microenvironment.
Methods
We developed a murine model of Cebpa mutant AML, where Cebpa mutant leukemic initiating cells (LICs) were injected into sub-lethally irradiated young mice, reproducibly leading to AML in 4 weeks. Flow cytometry was used to study the changes that occur to the abundance of BM niche during AML development. Molecular profiling of leukemic cells and BM stromal cells during leukemogenesis was performed, and the identified changes were overlaid with those occurring during physiological ageing.
Results
In our Cebpa mutant AML model, the 2-, 3- and 4-week time points represented 3 key stages of leukemia progression: i) an early expansion phase with no overt leukemia; ii) a late expansion phase with leukemia cells highly abundant in hematopoietic organs, and iii) a terminal phase where normal BM hematopoiesis is displaced (Figure 1A). As most AML cells occupy the central BM (CBM), we focused on CBM vascular endothelial cells (VECs) and perivascular cells (PVCs). We observed a decrease in the number of CBM VECs in leukemic, compared to mock transplanted mice, which was similar to that observed during normal ageing. CBM VECs and PVCs were profiled by RNA sequencing at the 3 key time points after LIC transplantation, with cells from mock transplanted mice profiled as controls. In CBM VECs, we observed a significant overlap between the genes and process networks altered during ageing and those altered at week 3 of AML (Figure 1B). In addition, several cytokines produced by leukemic cells were capable of expanding myeloid progenitors (the closest normal counterpart of LIC) in vivo. These cytokines were also upregulated in aged BM stromal cells, consistent with the aged BM microenvironment sharing characteristics with that adapted to AML (Figure 1C-D). This hypothesis was confirmed when we observed leukemia engraftment only in non-irradiated aged mice injected with Cebpa mutant LICs but not in young mice (Figure 1E).

Conclusion
Molecular and functional similarities exist between the BM microenvironment generated during leukemia progression and physiological ageing. These similarities may contribute to increased AML incidence and progression by providing support for the expansion of myeloid progenitors, including LICs.
Keyword(s): AML, Microenvironment


