
Contributions
Abstract: EP374
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
hnRNP K is a ribonucleoprotein involved in processing hnRNAs into mature mRNAs, transcription, and translation control. hnRNP K participates in the nuclear-cytoplasmic shuttling and transmission of information from the nucleus to the cytoplasm. In previous studies, we noticed that HNRNPK overexpression is linked with acute myeloid leukaemia’s (AML) poor prognosis, which is also related to an oncogenic event, i.e. Nucleolar Stress Response (NSR) resistance. Thus, our objective is to decipher the underlying molecular mechanism by which hnRNP K is related to AML’s poor outcomes.
Aims
Our aim is to test the impact on proliferation and tumour development of hnRNP K overexpression due to the generation of NSR resistance
Methods
Clinical bone marrow samples from AML patients with high and low levels of hnRNP K (n=415) were used to study HNRNPK protein expression. Additionally, we analyzed HNRNPK copy number by FISH (n=205). Moreover, reverse-phase protein array (RPPA) of AML patients was performed and showed HNRNPK and C-MYC overexpression correlation. hnRNP K overexpression mice models, hnRNP KTg/EIIa-Cre and hnRNP KTg/UBC-Cre were used as validation of human samples. Molecular analysis (RT-qPCR, WB and Confocal Microscopy) of hnRNP K, c-Myc, B23 and NPM1 were performed using Mouse Embryonic Fibroblast (MEFs) from our mice models. Also, we analyzed protein synthesis capacity (OPP assay) and NSR sensors by confocal microscopy. Finally, viability and dose-response assays with genotoxic compounds were performed using trypan blue and WST-1
Results
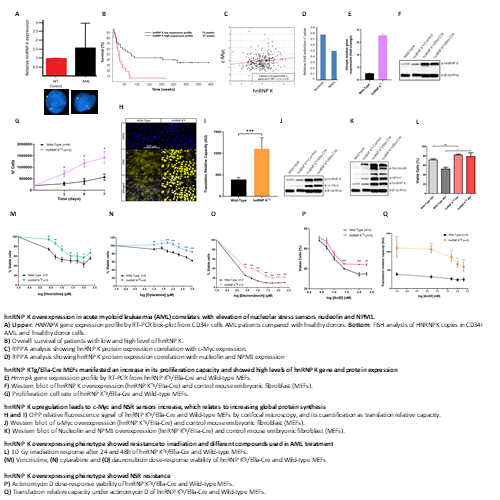
AML patient samples revealed an amplification in the copy number of HNRNPK and the consequent gene overexpression and poor outcomes. Moreover, HNRNPK overexpression correlates with c-MYC and NSR sensors (B23 and C23) overexpression (Fig. A, B, C, D). MEFs showed gene and protein high levels of hnRNP K, which correlates with an increase in its proliferation capacity (Fig. E, F, G). Molecularly, this increase leads to c-Myc and NSR sensors upregulation, contributing to increasing global protein synthesis (Fig. H, I, J, K). In addition, these cells showed resistance to irradiation and genotoxic compounds such as vincristine, cytarabine and daunorubicin (Fig. L, M, N, O). Furthermore, this hnRNP K upregulated phenotype manifests NSR resistance when we exposed these MEFs to Actinomycin D. This event could correlate with an increase in NSR sensors such as B23 and C23 (Fig. P, Q).
Conclusion
HNRNPK is amplified and overexpressed in AML patients, which manifest less survival than patients without this upregulation. Moreover, hnRNP K regulates c-Myc that triggers the global translation process. Therefore, the c-Myc upregulation leads to an increase in ribosome biogenesis and function, enhancing global translation. In addition, overexpression of hnRNP K drives B23 and C23 increase, which could establish a hyperactivated nucleolus. These events evoke an oncogenic event known as “Nucleolar Stress”. Moreover, hnRNP K overexpression showed NSR resistance. Super-nucleolus drives the NSR resistance that promotes the chemo-resistance to different AML treatments, explaining these poor outcomes. Thus, the development of a specific hnRNP K inhibitor would potentially open the door to new treatments of AML patients that do not respond to treatment due to hnRNP K overexpression.
Keyword(s): Acute myeloid leukemia, Drug resistance
Abstract: EP374
Type: E-Poster Presentation
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
hnRNP K is a ribonucleoprotein involved in processing hnRNAs into mature mRNAs, transcription, and translation control. hnRNP K participates in the nuclear-cytoplasmic shuttling and transmission of information from the nucleus to the cytoplasm. In previous studies, we noticed that HNRNPK overexpression is linked with acute myeloid leukaemia’s (AML) poor prognosis, which is also related to an oncogenic event, i.e. Nucleolar Stress Response (NSR) resistance. Thus, our objective is to decipher the underlying molecular mechanism by which hnRNP K is related to AML’s poor outcomes.
Aims
Our aim is to test the impact on proliferation and tumour development of hnRNP K overexpression due to the generation of NSR resistance
Methods
Clinical bone marrow samples from AML patients with high and low levels of hnRNP K (n=415) were used to study HNRNPK protein expression. Additionally, we analyzed HNRNPK copy number by FISH (n=205). Moreover, reverse-phase protein array (RPPA) of AML patients was performed and showed HNRNPK and C-MYC overexpression correlation. hnRNP K overexpression mice models, hnRNP KTg/EIIa-Cre and hnRNP KTg/UBC-Cre were used as validation of human samples. Molecular analysis (RT-qPCR, WB and Confocal Microscopy) of hnRNP K, c-Myc, B23 and NPM1 were performed using Mouse Embryonic Fibroblast (MEFs) from our mice models. Also, we analyzed protein synthesis capacity (OPP assay) and NSR sensors by confocal microscopy. Finally, viability and dose-response assays with genotoxic compounds were performed using trypan blue and WST-1
Results
AML patient samples revealed an amplification in the copy number of HNRNPK and the consequent gene overexpression and poor outcomes. Moreover, HNRNPK overexpression correlates with c-MYC and NSR sensors (B23 and C23) overexpression (Fig. A, B, C, D). MEFs showed gene and protein high levels of hnRNP K, which correlates with an increase in its proliferation capacity (Fig. E, F, G). Molecularly, this increase leads to c-Myc and NSR sensors upregulation, contributing to increasing global protein synthesis (Fig. H, I, J, K). In addition, these cells showed resistance to irradiation and genotoxic compounds such as vincristine, cytarabine and daunorubicin (Fig. L, M, N, O). Furthermore, this hnRNP K upregulated phenotype manifests NSR resistance when we exposed these MEFs to Actinomycin D. This event could correlate with an increase in NSR sensors such as B23 and C23 (Fig. P, Q).

Conclusion
HNRNPK is amplified and overexpressed in AML patients, which manifest less survival than patients without this upregulation. Moreover, hnRNP K regulates c-Myc that triggers the global translation process. Therefore, the c-Myc upregulation leads to an increase in ribosome biogenesis and function, enhancing global translation. In addition, overexpression of hnRNP K drives B23 and C23 increase, which could establish a hyperactivated nucleolus. These events evoke an oncogenic event known as “Nucleolar Stress”. Moreover, hnRNP K overexpression showed NSR resistance. Super-nucleolus drives the NSR resistance that promotes the chemo-resistance to different AML treatments, explaining these poor outcomes. Thus, the development of a specific hnRNP K inhibitor would potentially open the door to new treatments of AML patients that do not respond to treatment due to hnRNP K overexpression.
Keyword(s): Acute myeloid leukemia, Drug resistance


